1. Borie F, Bouvier AM, Herrero A, et al. Treatment and prognosis of hepatocellular carcinoma: a population based study in France. J Surg Oncol. 2008; 98:505–509. PMID:
18932235.

2. Lau WY, Lai EC. Salvage surgery following downstaging of unresectable hepatocellular carcinoma--a strategy to increase resectability. Ann Surg Oncol. 2007; 14:3301–3309. PMID:
17891443.

3. Chen XP, Qiu FZ, Wu ZD, et al. Effects of location and extension of portal vein tumor thrombus on long-term outcomes of surgical treatment for hepatocellular carcinoma. Ann Surg Oncol. 2006; 13:940–946. PMID:
16788755.

4. Ng KK, Vauthey JN, Pawlik TM, et al. International Cooperative Study Group on Hepatocellular Carcinoma. Is hepatic resection for large or multinodular hepatocellular carcinoma justified? Results from a multi-institutional database. Ann Surg Oncol. 2005; 12:364–373. PMID:
15915370.

5. Chirica M, Scatton O, Massault PP, et al. Treatment of stage IVA hepatocellular carcinoma: should we reappraise the role of surgery? Arch Surg. 2008; 143:538–543. PMID:
18559745.
6. Ruzzenente A, Capra F, Pachera S, et al. Is liver resection justified in advanced hepatocellular carcinoma? Results of an observational study in 464 patients. J Gastrointest Surg. 2009; 13:1313–1320. PMID:
19418103.

7. Liu CL, Fan ST, Lo CM, Ng IO, Poon RT, Wong J. Hepatic resection for bilobar hepatocellular carcinoma: is it justified? Arch Surg. 2003; 138:100–104. PMID:
12511161.
8. Pawlik TM, Poon RT, Abdalla EK, et al. Hepatectomy for hepatocellular carcinoma with major portal or hepatic vein invasion: results of a multicenter study. Surgery. 2005; 137:403–410. PMID:
15800485.

9. Lau WY, Ho SK, Yu SC, Lai EC, Liew CT, Leung TW. Salvage surgery following downstaging of unresectable hepatocellular carcinoma. Ann Surg. 2004; 240:299–305. PMID:
15273555.

10. Tang ZY, Zhou XD, Ma ZC, et al. Downstaging followed by resection plays a role in improving prognosis of unresectable hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2004; 3:495–498. PMID:
15567731.
11. Torzilli G, Del Fabbro D, Palmisano A, Marconi M, Makuuchi M, Montorsi M. Salvage hepatic resection after incomplete interstitial therapy for primary and secondary liver tumours. Br J Surg. 2007; 94:208–213. PMID:
17149716.

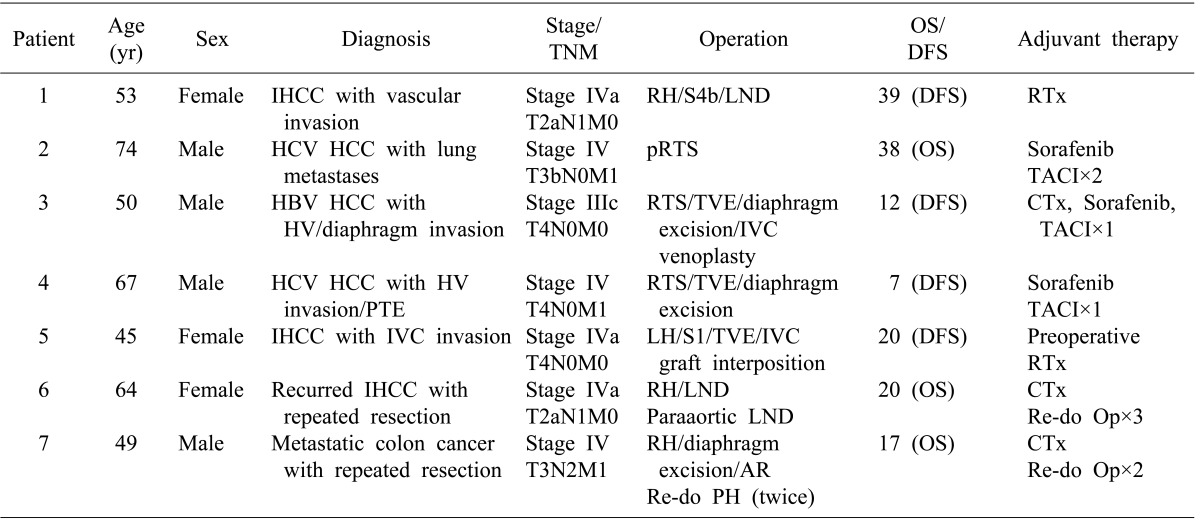
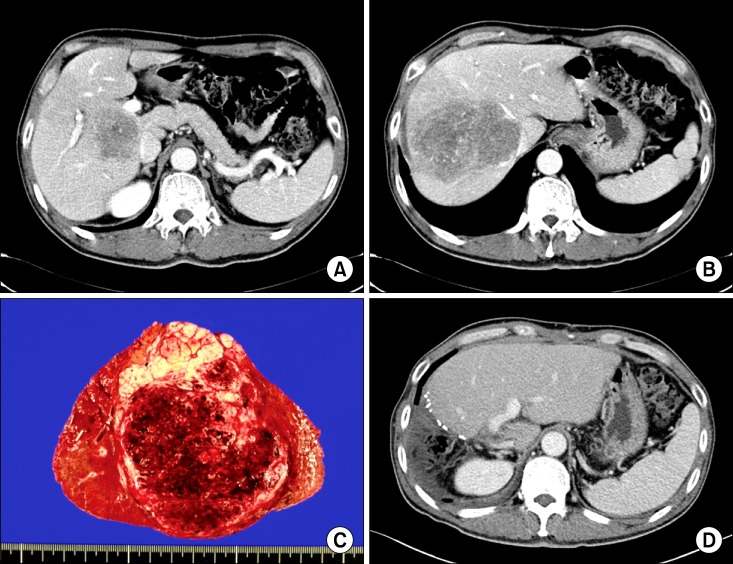
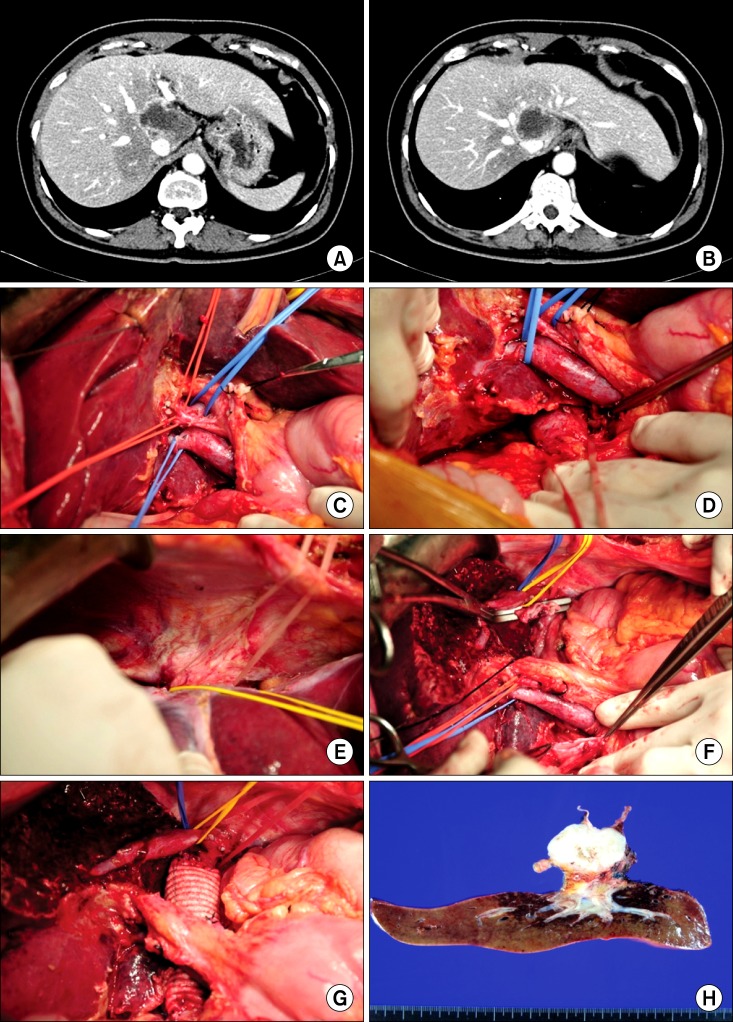
12. Lee JM, Park KM, Choi J, Chon SH, Hwang DW, Lee YJ. Palliative hepatectomy for advanced hepatocelluar carcinoma with multiple metastases: a case report. Korean J Hepatobiliary Pancreat Surg. 2009; 13:295–300.
13. Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003; 362:1907–1917. PMID:
14667750.

14. Liver Cancer Study Group of Japan. Primary liver cancer in Japan. Clinicopathologic features and results of surgical treatment. Ann Surg. 1990; 211:277–287. PMID:
2155591.
15. Shimada K, Sano T, Sakamoto Y, Esaki M, Kosuge T, Ojima H. Surgical outcomes of the mass-forming plus periductal infiltrating types of intrahepatic cholangiocarcinoma: a comparative study with the typical mass-forming type of intrahepatic cholangiocarcinoma. World J Surg. 2007; 31:2016–2022. PMID:
17687597.

16. Lang H, Sotiropoulos GC, Frühauf NR, et al. Extended hepatectomy for intrahepatic cholangiocellular carcinoma (ICC): when is it worthwhile? Single center experience with 27 resections in 50 patients over a 5-year period. Ann Surg. 2005; 241:134–143. PMID:
15622001.
17. Shimada M, Yamashita Y, Aishima S, Shirabe K, Takenaka K, Sugimachi K. Value of lymph node dissection during resection of intrahepatic cholangiocarcinoma. Br J Surg. 2001; 88:1463–1466. PMID:
11683741.

18. House MG, Ito H, Gönen M, et al. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010; 210:744–752. 752–755. PMID:
20421043.

19. Sakamoto Y, Fujita S, Akasu T, et al. Is surgical resection justified for stage IV colorectal cancer patients having bilobar hepatic metastases?--an analysis of survival of 77 patients undergoing hepatectomy. J Surg Oncol. 2010; 102:784–788. PMID:
20872814.

20. Jaeck D, Oussoultzoglou E, Rosso E, Greget M, Weber JC, Bachellier P. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg. 2004; 240:1037–1049. PMID:
15570209.

21. Ishiguro S, Akasu T, Fujimoto Y, et al. Second hepatectomy for recurrent colorectal liver metastasis: analysis of preoperative prognostic factors. Ann Surg Oncol. 2006; 13:1579–1587. PMID:
17003958.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download