Abstract
A 21-year-old woman presented gastrointestinal manifestation showing intermittent abdominal pain, nausea, and vomiting. An upper endoscopic examination showed round, elevated mucosa at the antrum of the stomach anterior wall. After CT scanning, a huge degenerated gastrointestinal stromal tumor was suspected. Subtotal gastrectomy with Billroth II anastomosis was performed. Histologically, pseudocystic degeneration of the heterotopic pancreas was confirmed. The patient showed eventful postoperative course except temporary dilated gastric emptying. The patient is doing well without any abnormal symptom at 8-month follow-up. This report is a rare case of gastric outlet obstruction caused by a pseudocyst originating from a heterotopic pancreas in the gastric antrum.
Go to : 
Heterotopic pancreas (HP) is a relatively uncommon condition that can occur at any age. It is ectopically localized pancreatic tissue with no anatomic and vascular connections to the true pancreas, and is found in 2-15% of all autopsies.1 Heterotopic pancreas is most frequently encountered in the duodenum (25-35%) and stomach (25-60%).23 The etiology of heterotopic pancreas is unknown; it is possible that during rotation of the foregut and fusion of the ventral and dorsal parts of the pancreas in early fetal life, small pieces of tissue become detached from the forming organ, leading to entrapment in a different location.4 Most patients with heterotopic pancreas are asymptomatic, but it can occasionally present as nausea, vomiting, and abdominal pain. Serious symptoms, such as peptic ulceration with severe gastrointestinal bleeding, malignant degeneration, pancreatitis, and pseudocyst, are rarely seen. This is a report on rare case of gastric outlet obstruction caused by a pseudocyst of a heterotopic pancreas in the gastric antrum.
Go to : 
A 21-year-old-female was admitted to our hospital with 6 month history of chronic epigastric pain, postprandial dyspepsia, and recurrent vomiting after meals. The patient had an unremarkable medical history. She had never smoked or consumed alcoholic beverages. She showed with stable vital signs. The mass was palpated with mild direct tenderness in the epigastric area. However, the rest of the physical examination was normal. The white blood cell count was 1,100/ul in hematological examinations, and blood biochemical findings were normal. All tumor markers, including alpha-fetoprotein, carbohydrate antigen 19-9 (CA19-9) and carcinoembryonic antigen (CEA), were in the normal range.
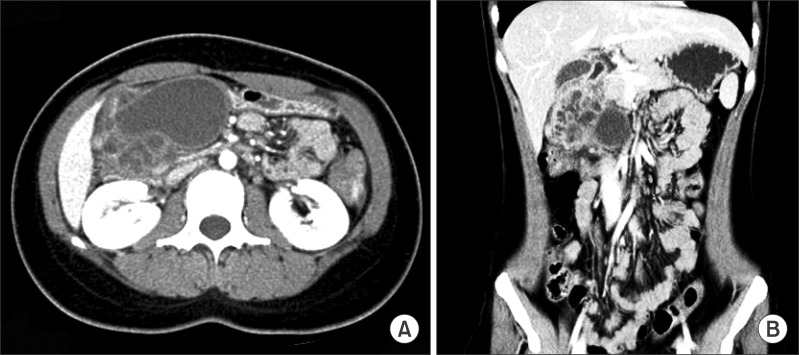
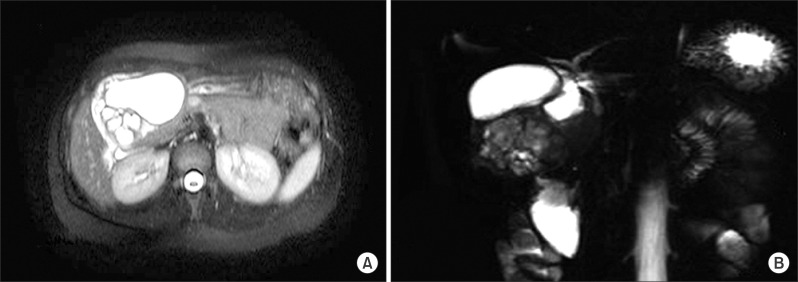
Gastroduodenoscopy showed chronic antritis and a submucosal lesion in the antero-inferior wall of the gastric antrum. Multi-slice spiral computerized tomography in the portal venous phase showed a 7.5 cm diameter well-defined lesion in the antero-inferior wall of the stomach. It contained a large cystic mass with likely cystic degeneration and multi-septated small cysts. The cystic mass showed no evidence of local invasion (Fig. 1). Magnetic retrograde cholangiopancreatography (MRCP) showed a large cystic lesion without connection to the common bile duct (CBD) or the pancreatic duct. The CBD showed mild dilatation due to compression of the cystic lesion (Fig. 2).
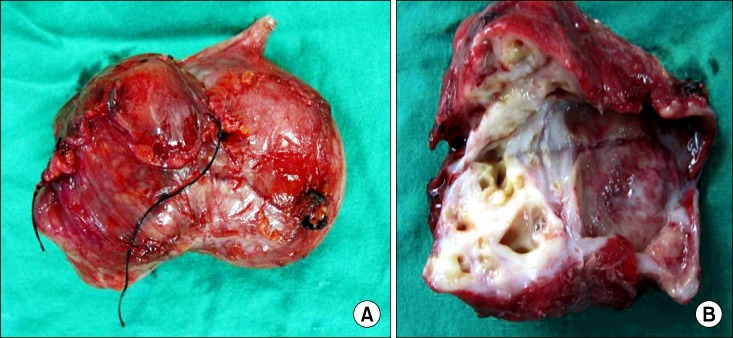
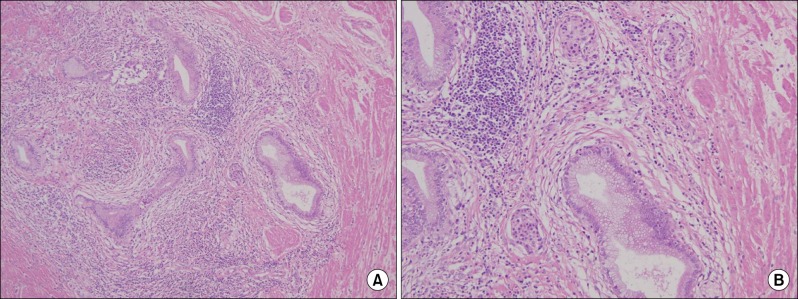
To rule out either gastrointestinal stromal tumor (GIST) or pancreatic pseudocyst, we decided to resect the cystic mass. During the operation, the cystic mass was not separated from the gastric antrum, but easily dissected from other surrounding tissues. We performed a wedge resection of the antrum of the stomach, including the cystic lesion, and a Billroth II gastrojejunostomy due to narrowing of the gastric outlet. During operation, the cystic mass was seen to contain yellowish fluid, likely pus, and no connection between the cystic mass and the mucosa of the resected antral wall. The wall of the cystic mass showed thickening with trabecular structures (Fig. 3). The resected specimen was 7.3 cm × 5.5 cm × 4 cm, and a histological examination showed benign glandular tissue within the muscle layer with the presence of islets of Langerhans and cystic changes of the ductular structure. There was no evidence of pancreatic acinar formation (Fig. 4). It was finally decided that mass was formed from cystic degeneration of heterotopic pancreas.
The patient was discharged 12 days after surgery. However, she was re-admitted with gastric outlet obstruction symptoms. Gastroscopy showed partial obstruction at the gastrojejunostomy site. She was treated with conservative management for 1 week and discharged with relief of symptoms. The patient did not suffer from other postoperative complication or recurrence of disease for postoperative 8 month.
Go to : 
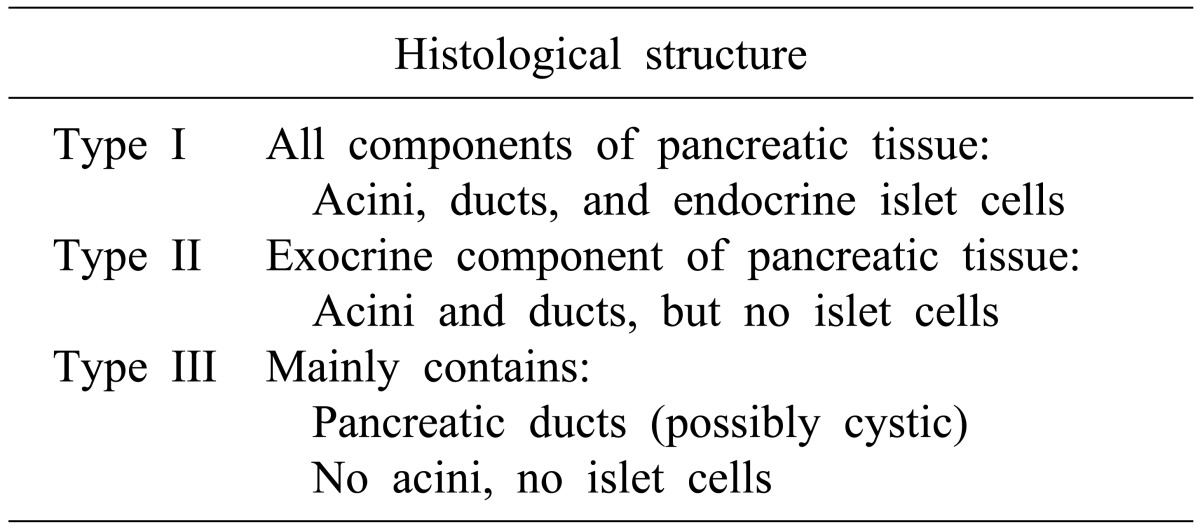
Heterotopic pancreas, also known as ectopic or aberrant pancreas, is defined as pancreatic tissue that lacks either anatomical or vascular communication with true pancreas and is reported in 0.6-13% of post-mortem examinations.4 Heterotopic pancreas can include all of the following: histological features, duct development, and islets of Langerhans. Distribution of the heterotopic pancreas tissue varies throughout the gastrointestinal tract. The most common site is the stomach, accounting for 25-38.2% of all patients with heterotopic pancreas.5 Most patients with heterotopic pancreas are asymptomatic.6 Reported symptoms include non-specific abdominal pain (45.5%), epigastric discomfort (12.0%), nausea and vomiting (9.6%), bleeding (8.0%), and others (24.5%).5 Complications caused by heterotopic pancreas are mechanical obstruction, cystic formation, acute inflammation, and malignant transformation.37 Histologically, heterotopic pancreas has been classified into three types by Heinrich (Table 1);8 the present case would be classified as type III.
Most patients with heterotopic pancreas had been diagnosed post-laparotomy on histological examination on histological examination of the resected specimen, usually suspected preoperatively as a gastrointestinal stromal tumor, lymphoma, or duodenal submucosal tumor. As such, this disorder is difficult to diagnose preoperatively despite diagnostic procedures such as abdominal ultrasonography, gastroduodenoscopy, and computed tomography910 According to one report,9 only 1 of 17 patients (6%) was considered to have heterotopic pancreas preoperatively. Present case also was initially diagnosed as gastrointestinal stromal tumor through preoperative imaging and endoscopic study until the postoperative pathology showed otherwise.
If gastric outlet obstruction caused by heterotopic pancreas occurs, as in present case, it can be treated by various operative procedures, including bypass gastroenterostomy or antrectomy with gastroduodenal anastomosis. Lymphadenectomy is not considered necessary as a lymphatic spread rarely occurs from a heterotopic pancreas or gastrointestinal stromal tumor.11 We performed antrectomy without lymph node dissection to resolve gastric outlet obstruction.
In conclusion, we herein present a rare case of gastric outlet obstruction caused by a pseudocyst originating from the heterotopic pancreas in gastric antrum. Although most of asymptomatic heterotopic pancreas usually is not clinical significance, it can be included in the differential diagnoses of gastric outlet obstruction caused by a submucosal gastric mass.
Go to : 
References
1. Jaffee R. The pancreas. In : Wigglesworth JS, Singer DB, editors. Textbook of fetal and perinatal pathology. 1991. Vol 2nd ed. Boston, Massachusetts: Blackwell Scientific;p. 1021–1055.
2. Mizuno Y, Sumi Y, Nachi S, et al. Acinar cell carcinoma arising from an ectopic pancreas. Surg Today. 2007; 37:704–707. PMID: 17643220.

3. Mulholland KC, Wallace WD, Epanomeritakis E, Hall SR. Pseudocyst formation in gastric ectopic pancreas. JOP. 2004; 5:498–501. PMID: 15536290.
4. Tolentino LF, Lee H, Maung T, Stabile BE, Li K, French SW. Islet cell tumor arising from a heterotopic pancreas in the duodenal wall with ulceration. Exp Mol Pathol. 2004; 76:51–56. PMID: 14738869.

5. Kaneda M, Yano T, Yamamoto T, et al. Ectopic pancreas in the stomach presenting as an inflammatory abdominal mass. Am J Gastroenterol. 1989; 84:663–666. PMID: 2729238.
6. Dolan RV, ReMine WH, Dockerty MB. The fate of heterotopic pancreatic tissue. A study of 212 cases. Arch Surg. 1974; 109:762–765. PMID: 4420439.
7. Song DE, Kwon Y, Kim KR, Oh ST, Kim JS. Adenocarcinoma arising in gastric heterotopic pancreas: a case report. J Korean Med Sci. 2004; 19:145–148. PMID: 14966359.

8. von Heinrich H. Ein beitrag zur histologie des sogen, akzessorichen pankreas. Virchows Arch Pathol Anat Physiol Klin Med. 1909; 198:392–401.
9. Hsia CY, Wu CW, Lui WY. Heterotopic pancreas: a difficult diagnosis. J Clin Gastroenterol. 1999; 28:144–147. PMID: 10078823.

10. Cho JS, Shin KS, Kwon ST, et al. Heterotopic pancreas in the stomach: CT findings. Radiology. 2000; 217:139–144. PMID: 11012436.

11. Cuschieri A. Laparoscopic gastric resection. Surg Clin North Am. 2000; 80:1269–1284. PMID: 10987035.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download