INTRODUCTION
Liver transplantation with preservation of the recipient vena cava (piggyback technique) has been performed as an alternative to conventional method. As another piggyback technique, side-to-side cavo-caval anastomosis has been proposed. Outflow disturbance or obstruction of the vena cava in the early period after liver transplantation is associated with high morbidity and mortality. These complications occur more frequently when the piggyback technique is used.
12
In deceased-donor liver transplantation (DDLT) with a classic piggyback technique, it was reported venous outflow obstruction with an incidence of 4.6%, and 40% of these patients required re-transplantation.
2 In DDLT with a modified piggyback technique, it was also reported an incidence of obstruction of 1.4%.
1 Another report presented that the incidence of outflow obstructions in the classic piggyback technique, the end-to-side technique, and the modified piggyback technique were 2.2%, 6.6%, and 0.7%, respectively.
3 Reported incidences of hepatic venous outflow occlusion following living-donor liver transplantation (LDLT) range from 3.9% to 16.6%.
4 There are various treatment methods for outflow obstruction, including endoluminal anastomotic dilation.
3
We have used a side-to-side piggyback technique (modified piggyback technique) for venous outflow reconstruction. We had one case of DDLT using a modified piggyback technique recently, but he had to undergo inferior vena cava stenting after operation. We did not find any reports of inferior vena cava stents for the modified piggyback technique in literature in English.
Herein we report a case of DDLT using the modified piggyback technique, who received inferior vena cava stent due to outflow disturbances of the reconstructed orifice of the vena cava.
Go to :

CASE
A 50-year-old male was admitted to the hospital due to jaundice for 7 days. He had been diagnosed with liver cirrhosis from chronic viral hepatitis B for 8 years. He had been a heavy smoker (20 pack-years), but had quit 20 years before. The patient had no history of diabetes mellitus, essential hypertension, hyperlipidemia, heart disease, trauma, vasculitis, and connective tissue disease. His blood pressure was 120/80 mmHg, and pulse rate was 80 /min. His body weight was 78.2 kg, and his height was 160 cm. All his close family members were infected with viral hepatitis B. Physical examination showed icteric sclera, but no anemic conjunctiva. He had no tenderness or rebound tenderness of the abdomen. Routine laboratory tests showed total bilirubin of 32 mg/dl, BUN/Cr of 11.4/0.5 mg/dl, and prothrombin time of INR 6.39. Preoperative abdominal computed tomography (CT) showed liver cirrhosis. Model for end-stage liver disease (MELD) score was 40, and his mental status was hepatic encephalopathy of stage III to IV.
Regarding the operation, both ends of the graft inferior vena cava were closed with staplers. After tangential clamping of the inferior vena cava, the recipient cava was incised approximately 25 mm. Venous outflow reconstruction was performed using a side-to-side cavo-caval anastomosis (modified piggyback) method. The total operation time was 8 hour 30 minutes, cold ischemic time was 6 hour 35 minutes, anhepatic time was 72 minutes, and warm ischemic time was 38 minutes. His condition was stable enough to be moved to the general ward on postoperative day 5. But, he showed psychotic activity on postoperative day 7. He kept refusing to take the medicine, and spat out his immunosuppressive agents.
On postoperative day 9, he developed symptoms of abdominal discomfort, and abnormal liver functions: serum total bilirubin of 6.2 mg/dl and AST/ALT of 297/597 IU/L. Doppler ultrasound examination on postoperative day 4 showed mono-phasic waveforms of the hepatic vein (
Fig. 1). Dynamic CT was performed on postoperative day 6 and showed focal narrowing at the cavo-caval anastomosis site and mild luminal narrowing at the portal vein anastomosis site (
Fig. 2).
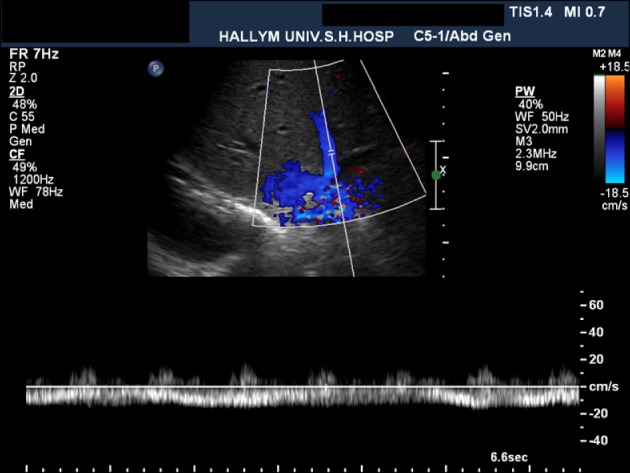 | Fig. 1Doppler sonogram obtained on the fourth postoperative day. The spectral Doppler waveform of the middle hepatic vein shows a mono-phasic flow pattern suggesting hepatic outflow stenosis.
|
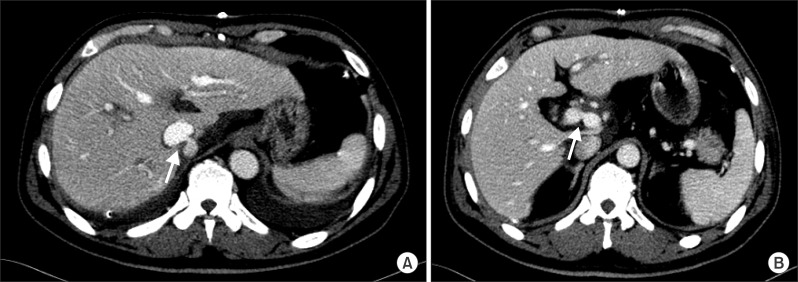 | Fig. 2CT scan images of portal phase at postoperative day 6. (A) There is focal luminal narrowing (arrow) at the side-to-side anastomotic site of the intrahepatic inferior vena cava. (B) Mild focal luminal narrowing (arrow) is also seen at the end-to-end anastomotic site of the main portal vein.
|
Liver biopsy was performed on postoperative day 10, and there was no evidence of acute allograft rejection. On postoperative day 11, he underwent hepatic venogram to check the pressure gradient under the suspicion of hepatic outflow obstruction. The venogram showed stenosis of the cavo-caval anastomosis with a pressure gradient of 12 mmHg. A SMART nitinol stentⓒ (12 mm in diameter and 4 cm in length, self-expandable metallic stent, Cordis, J & J Medical Systems, Miami Lakes, FL, USA) was inserted in the stenotic site of the inferior vena cava. After the procedure, the pressure gradient between the right hepatic vein and the recipient inferior vena cava decreased from 12 mmhg to 2 mmHg (
Fig. 3). Another stent was inserted into the portal vein because of mild anastomotic stenosis. A follow-up CT obtained 1 day after stent insertion demonstrated resolution of the stenosis in the cavo-caval anastomotic site and the portal vein anastomotic site (
Fig. 4). He was discharged from hospital on postoperative day 23 with serum total bilirubin of 2.3 mg/dl and AST/ALT of 69/239 IU/L, and without any other symptoms. A liver function test 6 months after liver transplantation showed total bilirubin of 0.8 mg/dl and AST/ALT of 19/15 IU/L.
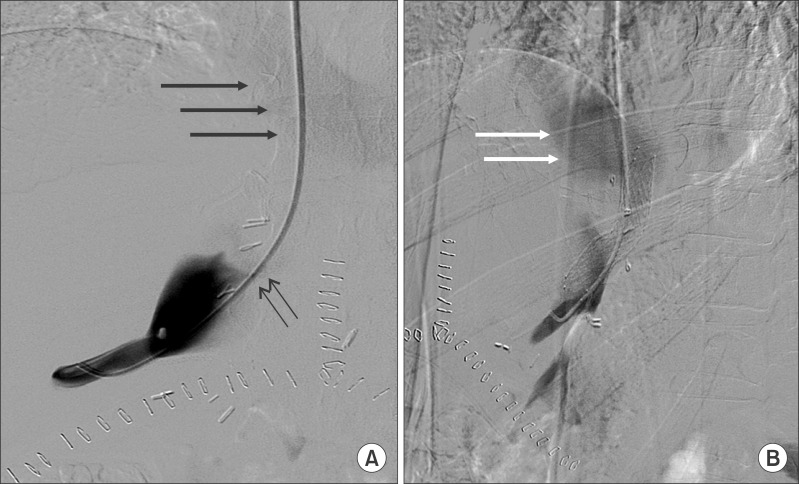 | Fig. 3Interventional stent placement at the cavo-caval anastomosis site on postoperative day 11. (A) Right hepatic venography shows contrast pooling in the donor inferior vena cava (IVC) and faint filling of the recipient IVC and right atrium (thick arrows) due to tight stenosis (thin arrows). (B) Hepatic venography after stent insertion across the stenosis (left anterior oblique 44° view). Early contrast flow into the right atrium (arrows) is visible. The pressure gradient between the right hepatic vein and the recipient IVC decreased from 12 mmHg to 2 mmHg after stent insertion.
|
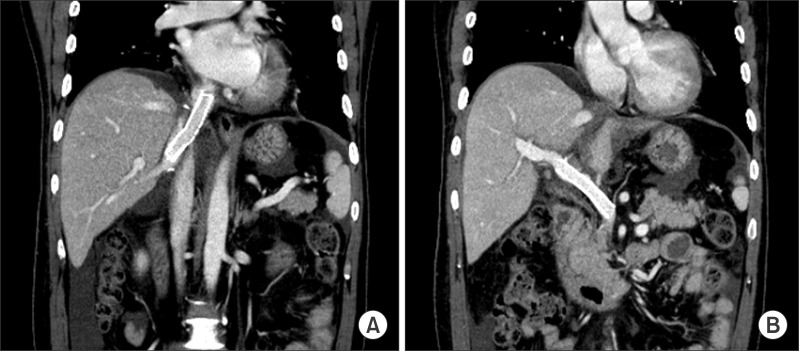 | Fig. 4Follow-up CT images at 1 day after stent insertion. (A) The CT image shows a patent stent without narrowing in the cavo-caval anastomosis. (B) The CT image reveals resolution of the stenosis at the portal vein anastomotic site after stenting.
|
Go to :

DISCUSSION
Multiple factors are known to play important roles in the outcome after liver transplantation. Among them, some are partly or completely related to the transplantation technique. The operative time, including the warm ischemic time and anhepatic time, hemorrhage, and hemodynamic instability are the most important ones. Additionally, it has been reported that the outflow is as important a factor as the inflow.
56
With the clinical establishment of the piggyback technique by Tzakis et al.,
7 some complications of conventional liver transplantation could be overcome. Their classic piggyback technique has several advantages over conventional liver transplantation, such as avoiding the use of extra-corporeal veno-venous bypass and inferior vena cava anastomosis. This leads to shortening of the total operation time and warm ischemic time, and a reduction in complications associated with dissection of the retrohepatic inferior vena cava, such as phrenic nerve injury and hemorrhage. In addition, the classic piggyback technique allows an improvement in intraoperative hemodynamic stability by maintenance of venous return, a reduction of intraoperative blood and fluid administration, improved postoperative renal function, better maintenance of oxygen delivery to tissues throughout the surgery, and a reduction in hospital stay length.
1 Moreover, the classic piggyback technique does not compress the inferior vena cava and does not cause kinking of the anastomosis because the anastomosis is located in the right and anterior portion of the recipient inferior vena cava.
8
There have been criticisms of the classic piggyback technique. Bismuth et al.
9 reported that his method had an advantage over the classic piggyback technique because the classic piggyback technique could cause a low flow area followed by thrombosis and a pendulous graft with downward displacement. The classic piggyback technique has been associated with some disadvantages and complications, including hepatic venous outflow obstruction and thrombosis in up to 10%, because of the inappropriate size of the hepatic vein outlet, which results in venous congestion of the liver allograft and post-transplant ascites, and even Budd-Chiari syndrome. These problems led to technical modifications of the classic piggyback technique, in which a side-to-side cavo-caval anastomosis was proposed.
10 This reduces the risk of upper caval outflow obstruction and Budd-Chiari-like syndrome, permits the avoidance of caval occlusion, and almost eliminates the indications of veno-venous bypass for patients who do not tolerate the inferior vena cava cross-clamping test.
111 One of the other advantages of the modified piggyback is the ease of retransplantation. If retransplantation is necessary, the non-functional liver can be conveniently removed with preservation of the anastomosis and a cuff of the donor's inferior vena cava. The caval anastomosis can be performed between the cuff and the second donor's inferior vena cava in a side-to-side fashion.
112
Outflow obstruction has been variably attributed to the creation of too small outflow tract, twisting of the anastomosis, compression of the liver on the inferior vena cava, kinking of the hepatic veins due to long graft suprahepatic inferior vena cava, other technical errors, abnormalities of the inferior vena cava, and adhesions between the liver and the retrohepatic inferior vena cava.
113 But most of all, a voluminous donor graft or an enlarged caudate lobe becomes the anatomic limitation that is important in situations that limit the use of caval preserving procedures. In the modified piggyback technique, the incision on the inferior vena cava should have enough distance from the hepatic veins and the closed cranial part of the donor inferior vena cava (approximately 10 mm), to avoid graft outflow obstruction and a functional Budd-Chiari-like syndrome.
1
There are various treatment modalities for outflow obstruction, such as simple rotation of the graft, caval anastomosis reconstruction, omentoplasty, diaphragmatic placement of the graft, a second caval anastomosis, endoluminal anastomotic dilation, conversion to standard orthotopic liver transplantation, and retransplantation.
3 In traditional conventional anastomosis which does not use the piggyback technique, stent insertion has been reported for the treatment of outflow obstruction.
They were concerned about the risk of disruption of a relatively fresh anastomosis during balloon angioplasty because a hepatic venous outflow abnormality was diagnosed in the early post-transplant period (less than 4 weeks). Balloon angioplasty frequently needed stent placement or surgical reposition because of recurrences of outflow obstruction in the late period.
4 Navarro et al.
3 reported that of 1,361 liver transplantation cases, 21 had outflow obstruction after the operation. They underwent multiple treatments including endoluminal anastomotic dilatation, but did not mention stent insertion. Thus we think they did not perform interventional stent insertion for those patients.
We also inserted a portal vein stent in present case. The portal vein stent was not the focus in this study, but there have been a few cases of portal vein stents after liver transplantation, and they were performed not infrequently.
14
Despite the widespread acceptance of piggyback technique, many surgeons have performed numerous diverse incision length of inferior vena cava, and they have difficulties in deciding what incision length of inferior vena cava of the donated liver. One underlying reason for this may be the rare studies of the proper incision length of the inferior vena cava. Mehrabi et al.
1 insisted that it was performed as 60 mm approximately, but they did not identify the specific reason to do that. In the clinical situation, it is not always so easy to make 60 mm incision length of inferior vena cava. Although Belghiti introduced the modified piggyback technique for the first time, recently he stopped doing his modified piggyback technique due to the problem of inferior vena cava exposure.
15
The normal diameter of the inferior vena cava is reported to be 30 mm × 20 mm.
16 Some surgeons insist the incision length of the inferior vena cava the same as its diameter. In present case, the incision length of the inferior vena cava was 25 mm. Some experiments suggested that appreciable changes in pressure and flow do not occur until the cross-sectional area of a vessel has been reduced by more than 75%. The reduction in cross-sectional area is symmetric, and corresponds to at least a 50% reduction in vessel diameter.
1718 We could not find a reliable incision length of the inferior vena cava, searching for articles on liver transplantation. We carefully suggest that we have to decide on the incision length of the inferior vena cava based on that golden rule of the experimental vascular work above-mentioned.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download