Abstract
Backgrounds/Aims
Because of low incidence rates, there have been few reports on the patterns of and risk factors for recurrence after curative resection of the ampulla of Vater (AoV) cancer. The aim of this study was to characterize patterns of recurrence and to evaluate risk factors affecting survival rates and recurrence after curative resection.
Methods
Medical records of 181 patients who had undergone pancreaticoduodenectomy with curative intent for AoV adenocarcinoma between December 1994 and March 2010 at Samsung Medical Center were retrospectively reviewed. Factors influencing on overall survival rate, recurrence rates, and recurrence patterns were analyzed.
Results
Lymph node metastases and high preoperative serum carcinoembryonic antigen (CEA) level >5 ng/ml were identified as independent factors affecting overall survival (p=0.006, p<0.001, respectively). Among the 181 patients, 69 developed local or distant recurrence within 3 years after curative resection. Lymph node metastasis, preoperative serum CEA level >5 ng/ml, and total bilirubin level >1.5 mg/dl were identified as independent prognostic factors of recurrence after curative resection (p=0.008, p<0.001, p=0.003, respectively).
Conclusions
AoV adenocarcinoma has a better prognosis than other periampullary carcinomas, but still has a high recurrence rate, especially during the first three years after curative radical resection. Therefore, careful follow-up is needed during the first 3 years, especially for the higher risk group. Further study of adjuvant therapy to decrease recurrence after curative resection is now warranted.
Go to : 
Carcinoma of the ampulla of Vater is a rare disease that accounts for 0.6% of gastrointestinal malignancies and 6% of periampullary cancers.12 It accounts for 20% of resected periampullary cancers,34 and has been known to have a better prognosis than pancreatic carcinoma and bile duct cancer.24 Curative resection is the most effective treatment to date. High curative resection rate more than 80% has been achieved with development of effective diagnoses and surgical methods as well as pre- and post-operative management.25 The complication rate related to surgery has been reported to be less than 10%.67 To improve the survival rate, other treatments, such as chemotherapy and radiotherapy, have been used in addition to surgery. Although these treatments have been reported to reduce the recurrence rate of the cancer and to increase the quality of life of the patients, no study reporting a significant improvement in the survival rate has been conducted to date.8910 Recently, local resection or endoscopic resection of the ampulla alone was done for patients at early stages or with a high surgical risk, but the prognosis is a subject of controversy.11121314
Because the incidence of ampullary cancer is relatively low compared to that of other periampullary cancers, reports on prognosis and recurrence of this disease are only a few. In fact, because of this, studies on recurrence and predictors of recurrence are even rarer. At present the recurrence rate has been reported to be 26-46% after curative resection. Therefore, further studies on recurrence and predictive factors after resection, and on adjuvant treatments for recurrence prevention are now required.151617
In present study, the results of curative resection for adenocarcinoma of the ampulla of Vater conducted over the past 15 years were analyzed to determine survival rates, survival-predictive factors, and recurrence patterns.
Go to : 
This study was conducted on 224 patients who underwent curative resection due to adenocarcinoma of the ampulla of Vater in Samsung Medical Center between December 1994 and June 2009, and who were discharged. This did not include patients who died from complications related to the surgery. Eight cases where conventional surgical treatment was performed due to hepatic metastasis were excluded. Of the 224 patients, 181 were selected for study. We excluded 42 patients who were found to have 11 lymph nodes or less in the postoperative pathologic examination to reduce errors related to lymph node metastasis, and 1 patient with inaccurate medical records.
Clinical data from the patient medical records were retrospectively analyzed, and follow-up was conducted via the outpatient clinic and telephone surveys. The follow-up was conducted for tumor markers such as carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9), and radiological evaluations such as chest X-ray examination, abdominal computed tomography (CT), and ultrasonography. If recurrence was suspected, it was confirmed via follow-up or additional examinations, such as magnetic resonance imaging (MRI) and positron emission tomography (PET), and the date of recurrence was considered the date when the abnormal findings were first observed. Outpatient follow-up was conducted every three to six months. If no recurrence was observed for five years, the follow-up examination was thereafter conducted annually.
Go to : 
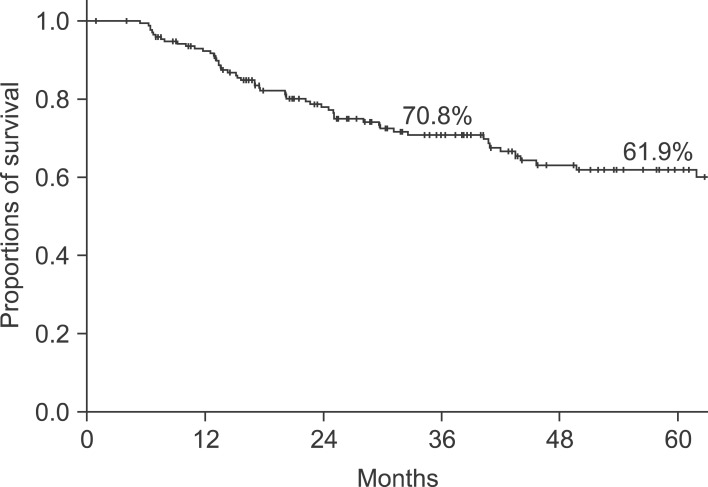
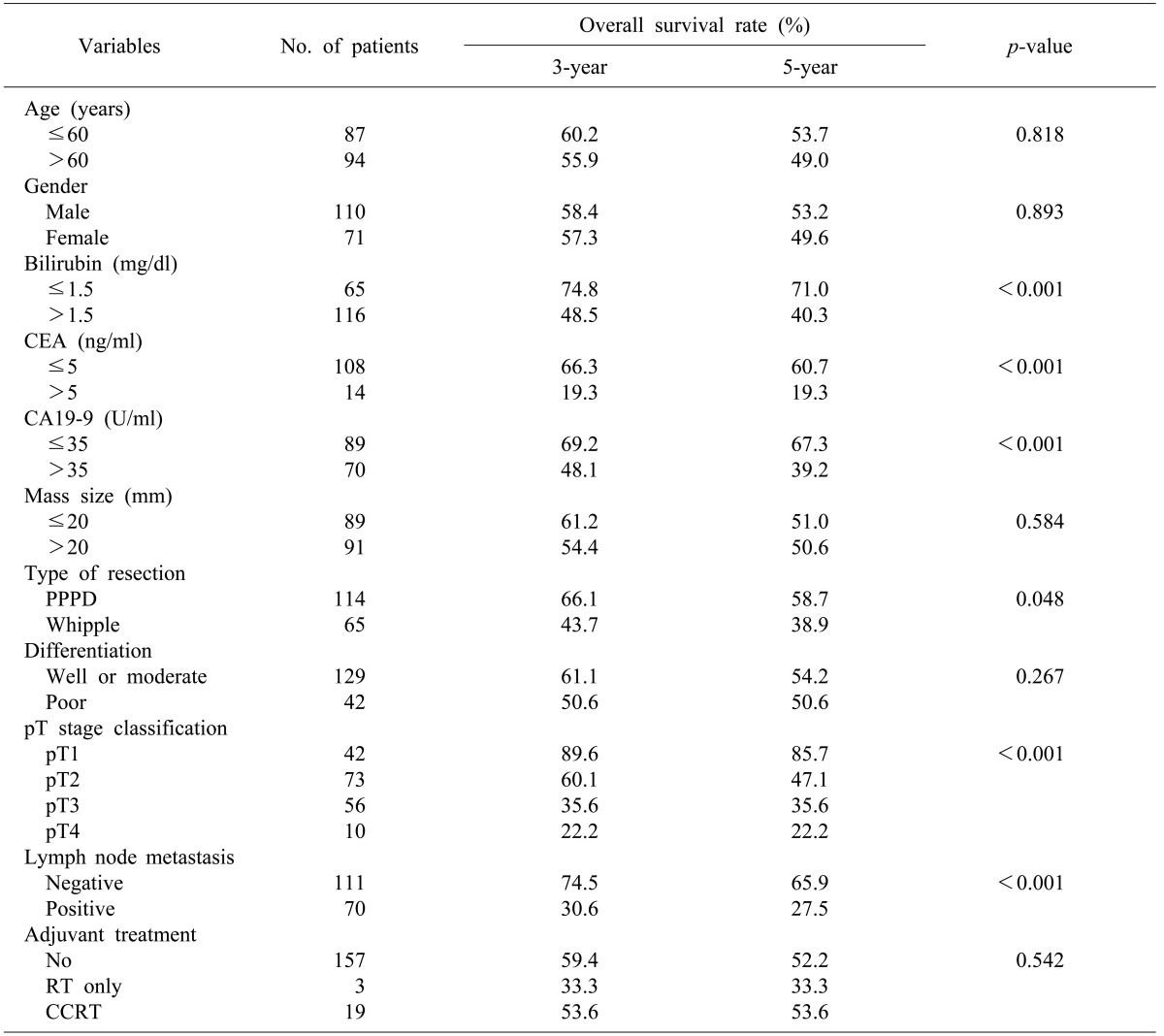
The study subjects included 181 patients (male: 110; female: 71) who underwent pancreaticoduodenectomy due to adenocarcinoma of the ampulla of Vater. The mean age of the patients was 58.9 years (age range: 32-84 years) at diagnosis. Pylorus-preserving pancreaticoduodenectomy was performed for 116 cases, and Whipple's procedure for 65 cases. Total pancreatectomy was conducted in one case with concurrent necrotizing pancreatitis and pylorus-preserving pancreaticoduodenectomy and right hemicolectomy were simultaneously conducted in one case with suspected invasion into the transverse colon (Table 1). Sixty two patients died during the follow-up period after the curative surgery. Among these, 56 had cancer and 6 showed no association with cancer or an unclear relevance to cancer. Except for the 6 patients who died for non-cancer related reasons, the 3 and 5 year survival rates for 175 of the 181 patients were found to be 70.8 and 61.9%, respectively. The median survival duration was 29.7 months (mean: 39.6 months; range: 0.9-162.6 months) (Fig. 1). Of the 62 patients who died of cancer, four had survived for more than 5 years. The 5 year survival rates according to AJCC disease stage were as follows: 96.7 and 62.0% in stages Ia and Ib, respectively; 71.4 and 41.1% in IIa and IIb, respectively; and 25.0% in III.
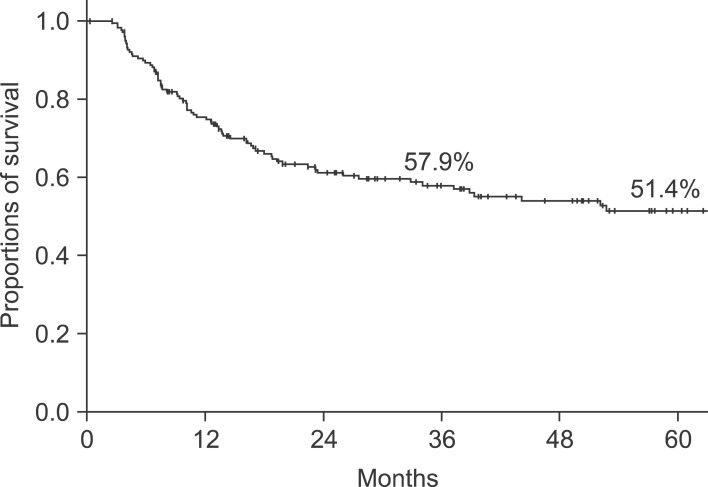
Disease recurrence occurred in 77 patients (42.5%) of the 181 during the follow-up period. In patients with recurrence, the median follow-up period was 19.9 months (mean: 15.2 months; range: 2.6-73.2 months). Sixty nine patients (89.6%) showed recurrence within three years after surgery, which was a majority of the 77 patients. Six patients showed recurrence between 3 and 5 years after surgery, and two patients showed recurrence more than 5 years after surgery. The disease-free 3- and 5-year survival rates of all patients who underwent surgery were 57.9 and 51.4%, respectively (Fig. 2). In patients who died of cancer, the median and mean survival durations after recurrence were 6.9 and 10.1 months, respectively (range: 1.0-43.8 months). When the 77 patients with recurrence were classified according to recurrence type, local recurrence was the most frequently occurring type - it was found in 50 patients (64.9%).
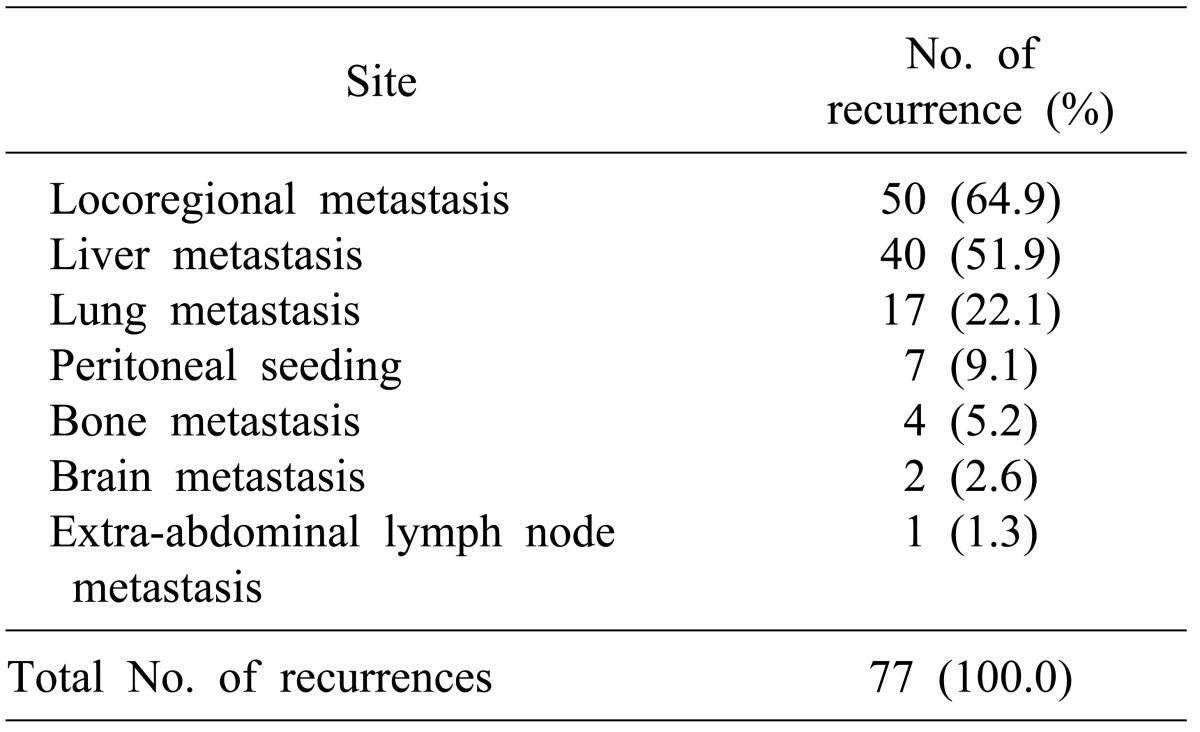
Frequencies of distant metastases by organ were: hepatic metastasis in 40 (51.9%), pulmonary metastasis in 17 (22.1%), peritoneal metastasis in 7 (9.1%), bone metastasis in 4 (5.2%), brain metastasis in 2 (2.6%), and pleural and subcutaneous tissue metastasis around the surgical site in 1 (Table 2).
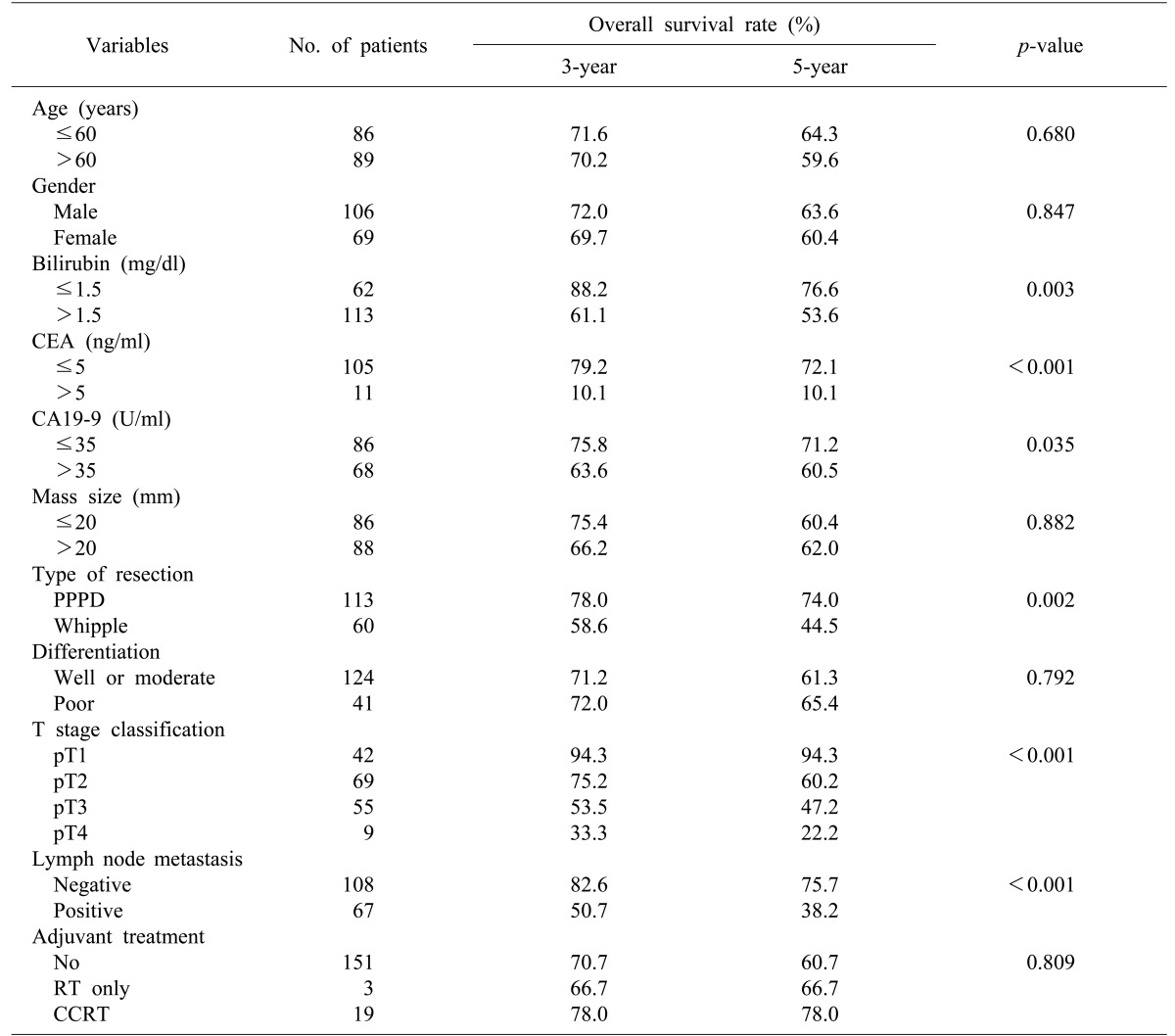
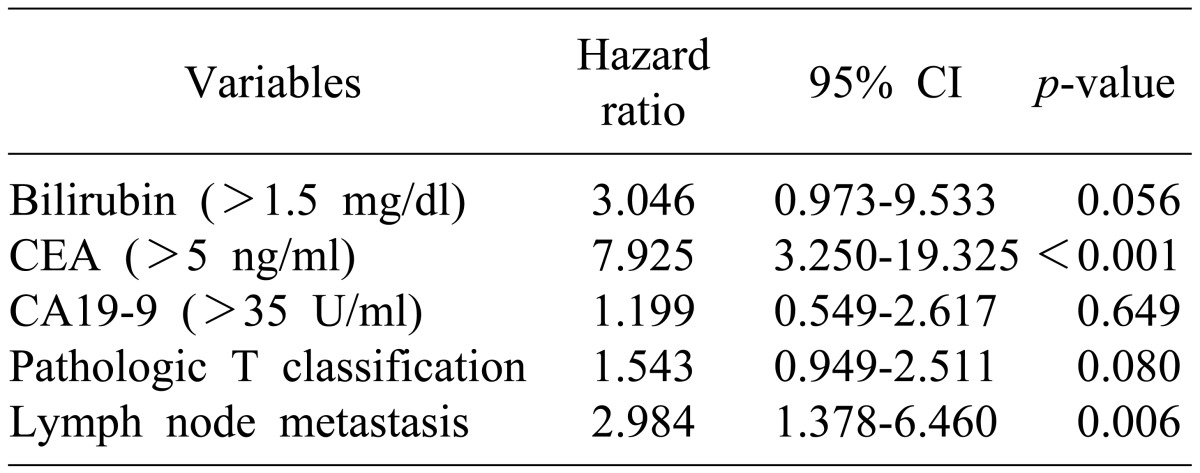
In a univariate analysis of the predictive factors for survival, the following were determined to be factors associated with the prognosis (Table 3): serum total bilirubin >1.5 mg/dl, CA19-9 >35 U/ml, CEA >5 ng/ml, tumor invasion depth, lymph node metastasis, and surgical method. In a multivariate analysis of predictive factors for survival, CEA >5 ng/dl (p<0.001) and lymph node metastasis (p=0.006) were statistically significant (Table 4).
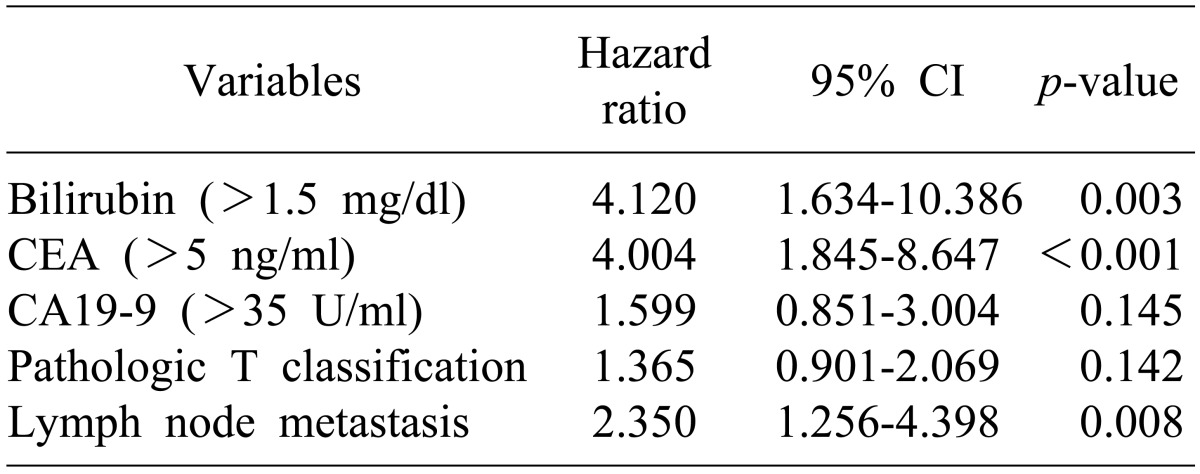
Results of univariate analysis for predictive factors for disease-free survival were the same as for univariate analysis of the predictive factors for survival (Table 5). In a multivariate analysis of the predictive factors for disease-free survival, the following were shown to be factors predicting recurrence (Table 6): CEA concentration >5 ng/dl (p<0.001), lymph node metastasis (p=0.008), and total bilirubin concentration >1.5 mg/dl.
Go to : 
Carcinoma of the ampulla of Vater accounts for approximately 6% of periampullary cancers2 and has a better prognosis.3418 The 5-year survival rate after curative resection was 40-65%,35615161819202122 and the recurrence rate during the follow-up period was 26-42%.151617 R0 resection is achieved in more than 80% of cases of carcinoma of the ampulla of Vater, which is higher than that of other periampullary cancers.25 Due to the low recurrence rate of ampullary cancer, the number of patients used in studies is small. Further, most of such studies have focused on the survival rate and its prognostic factors; there have been few studies on recurrence rates and factors that predict recurrence.
Prognostic factors related to survival after curative resection in carcinoma of the ampulla of Vater are tumor invasion depth,61921 lymph node metastasis,6151819 perineural invasion,15 histopathological feature,518 and lymph node metastasis rate (LNR: number of metastatic lymph nodes/number of lymph nodes resected or examined).23 In this study, lymph node metastasis was shown to be an independent prognostic factor related to survival rate. Furthermore, the lymph node metastasis rate in patients with carcinoma of the ampulla of Vater seems to be associated with the survival rate (Table 7). Studies on the correlation of lymph node metastasis with prognosis have been conducted. Falconi et al.23 reported that the prognosis was good when the number of lymph nodes identified in the lymph node dissection was 16 or more, and that poor prognosis was found as LNR increased. Bogoevski et al.24 reported that lymph node micrometastasis was found in patients diagnosed with N0 in the postoperative pathologic examination via immunohistochemical staining, and that the prognoses of the N0 patients with lymph node micrometastasis were similar to those of the N1 patients.
Howe et al.2 and Carter et al.5 reported that in carcinoma of the ampulla of Vater, the pancreatobiliary type showed poorer prognosis than the intestinal type, and that the cancer type was an independent prognostic factor. Besides the studies on cancer of the ampulla of Vater, studies on periampullary cancer also reported that the pancreatobiliary type showed poorer prognosis, and that the biological behavior of the cancer was a more important predictive factor than the primary site.3 Particularly, Carter et al.5 reported that jaundice was more frequently found in the pancreatobiliary type than in the intestinal type. In this study, the total bilirubin concentration was shown to be an independent predictive factor of the disease-free survival rate. This result is likely to be attributable to the histological features of the primary tumor, but it was not thoroughly investigated in this study. Therefore, a further study of the relationship between tumor histological features and prognosis is required.
In this study, lymph node metastasis, high total bilirubin and high CEA before surgery were independent prognostic factors associated with recurrence. In other studies, lymph node metastasis,16 venous invasion,17 perineural invasion,17 and LNR23 were associated with recurrence. As shown herein, this study and previous studies had common factors associated with the survival and recurrence rates. This result indicates that the survival rate is strongly associated with the recurrence rate in carcinoma of the ampulla of Vater. In studies on recurrence after curative resection, the recurrence rate during the follow-up period was reported to be 26.8-43.6%. In this study, the recurrence rate during the follow-up period and the five-year disease-free survival rate were reported to be 42.5 and 51.4%, respectively, similar to those in the previous studies.15161725
An interesting result of this study was that the blood CEA concentration measured before surgery was an independent factor predicting the survival rate and the disease-free survival rate. In addition to this study, Todoroki et al.25 reported that CEA and CA19-9 were recurrencepredictive factors in a study conducted on 66 ampullary cancer patients who underwent surgical treatment. In this study, CA19-9 was found to be a factor predicting survival rate, which is consistent with results of studies conducted by Nakao et al.26 and Dorandeu et al.27 In this study, CA19-9 showed statistical significance in univariate analysis of the disease-free survival rate, whereas it did not show statistical significance in multivariate analysis (p=0.144). As the blood CEA concentration can be easily measured before surgery, it is likely to be useful for the prediction of prognosis and recurrence.
Liver metastases are the most common organ metastases, followed by lung metastasis.15161725 The results of this study are consistent with the results of the previous study: local recurrence >liver metastasis >lung metastasis. Tumor recurrence was shown to occur in 69 patients (88.5%) within 3 years after the surgery, in 6 patients (7.7%) between 3 and 5 years after the surgery, and in 2 patients (2.6%) more than 5 years after surgery. Lymph node metastasis was found in 2 patients (25%) among 8 patients with recurrence beyond 3 years after the surgery, which was markedly different from 46 patients (66.7%) among 69 patients with recurrence within 3 years after the surgery. This difference is likely to be attributable to micrometastasis, which is not found in patients without lymph node metastasis.
In surgical treatments for carcinoma of the ampulla of Vater, curative resection procedure such as pancreaticoduodenectomy is the usual. This procedure causes major surgical complications such as pancreatic fistula and pseudoaneurysm, and has a repetitively higher death rate compared to other surgical treatments. Recently, death from surgical complications has significantly decreased, but various methods are still being investigated to reduce or avoid surgery-related risks.
In some studies, local or endoscopic resection of the ampulla was selectively performed for early carcinoma of the ampulla of Vater, and 3- and 5-year survival rates were similar to those of curative resection.111213 Such trials seem to be useful in groups with a high surgical risk or with early lesions such as Tis and T1, but this is still controversial. Yoon et al.14 suggested that surgical treatment should be carefully chosen for early cancer due to its high risk of recurrence, and stated that it is difficult to replace pancreaticoduodenectomy with other treatments even in early cancer. Terasawa et al.28 investigated prognostic factors in T1 lesions, reporting that only lymph node metastasis was a significant factor. The aforementioned studies confirmed that curative resection is necessary to avoid poor prognosis and recurrence despite the surgical risk involved. Furthermore, the study by Bogoevski et al.24 on the correlation of lymph node micrometastasis with prognosis also confirmed the necessity of appropriate lymph node dissection.
Adjuvant chemotherapy after curative resection or various adjuvant treatments of metastatic lesions have been conducted. Some studies showed promising results, but no definite treatment method that increases the survival rate has been established to date.91029 Recent studies reported that chemotherapy or chemoradiation therapy based on 5-fluorouracil had a potential for reducing local recurrence and for improving survival duration.8930 In particular, Komatsu et al.30 reported that administration of 5-fluorouracil and Cisplatin into the liver cured the hepatic metastasis that occurred after curative resection, and that a state with no recurrence was maintained during the 30-month follow-up period. A continuous study is required to reduce the high recurrence rate of carcinoma of the ampulla of Vater under conservative treatments.
In conclusion, we found that lymph node metastasis is a powerful prognostic factor as well as a recurrence-predictive factor. Blood CEA, which can be easily measured prior to surgery, can be used as a marker for predicting the survival rate and disease-free survival rate after curative surgery. Further studies are required to investigate the utilization of CA19-9 or total bilirubin as a predictive factor of prognosis and recurrence. Comparing to other periampullary cancers, carcinoma of the ampulla of Vater has a higher survival rate but also a high recurrence rate after curative resection. These results support the necessity of proactive follow-up after surgery. In particular, precautions should be taken within 3 years after surgery. If recurrence is suspected, an evaluation process should be conducted for the possibility of local recurrence or liver or lung metastasis. Furthermore, a study on conservative treatments after surgery is required for better prognosis.
Go to : 
References
1. Albores-Saavedra J, Schwartz AM, Batich K, Henson DE. Cancers of the ampulla of Vater: demographics, morphology, and survival based on 5,625 cases from the SEER program. J Surg Oncol. 2009; 100:598–605. PMID: 19697352.

2. Howe JR, Klimstra DS, Moccia RD, Conlon KC, Brennan MF. Factors predictive of survival in ampullary carcinoma. Ann Surg. 1998; 228:87–94. PMID: 9671071.

3. Hatzaras I, George N, Muscarella P, Melvin WS, Ellison EC, Bloomston M. Predictors of survival in periampullary cancers following pancreaticoduodenectomy. Ann Surg Oncol. 2010; 17:991–997. PMID: 20108122.

4. Yeo CJ, Sohn TA, Cameron JL, Hruban RH, Lillemoe KD, Pitt HA. Periampullary adenocarcinoma: analysis of 5-year survivors. Ann Surg. 1998; 227:821–831. PMID: 9637545.
5. Carter JT, Grenert JP, Rubenstein L, Stewart L, Way LW. Tumors of the ampulla of Vater: histopathologic classification and predictors of survival. J Am Coll Surg. 2008; 207:210–218. PMID: 18656049.

6. Qiao QL, Zhao YG, Ye ML, et al. Carcinoma of the ampulla of Vater: factors influencing long-term survival of 127 patients with resection. World J Surg. 2007; 31:137–143. PMID: 17171495.

7. Allema JH, Reinders ME, van Gulik TM, et al. Results of pancreaticoduodenectomy for ampullary carcinoma and analysis of prognostic factors for survival. Surgery. 1995; 117:247–253. PMID: 7878528.

8. Kim K, Chie EK, Jang JY, et al. Role of adjuvant chemoradiotherapy for ampulla of Vater cancer. Int J Radiat Oncol Biol Phys. 2009; 75:436–441. PMID: 19394162.

9. Morak MJ, van der Gaast A, Incrocci L, et al. Adjuvant intra-arterial chemotherapy and radiotherapy versus surgery alone in resectable pancreatic and periampullary cancer: a prospective randomized controlled trial. Ann Surg. 2008; 248:1031–1041. PMID: 19092348.
10. Morak MJ, Pek CJ, Kompanje EJ, Hop WC, Kazemier G, van Eijck CH. Quality of life after adjuvant intra-arterial chemotherapy and radiotherapy versus surgery alone in resectable pancreatic and periampullary cancer: a prospective randomized controlled study. Cancer. 2010; 116:830–836. PMID: 20029974.
11. Woo SM, Ryu JK, Lee SH, et al. Feasibility of endoscopic papillectomy in early stage ampulla of Vater cancer. J Gastroenterol Hepatol. 2009; 24:120–124. PMID: 19032444.

12. Yoon SM, Kim MH, Kim MJ, et al. Focal early stage cancer in ampullary adenoma: surgery or endoscopic papillectomy? Gastrointest Endosc. 2007; 66:701–707. PMID: 17905011.

13. Demetriades H, Zacharakis E, Kirou I, et al. Local excision as a treatment for tumors of ampulla of Vater. World J Surg Oncol. 2006; 4:14. PMID: 16524478.

14. Yoon YS, Kim SW, Park SJ, et al. Clinicopathologic analysis of early ampullary cancers with a focus on the feasibility of ampullectomy. Ann Surg. 2005; 242:92–100. PMID: 15973106.

15. Chiche L, Alkofer B, Parienti JJ, et al. Usefulness of follow-up after pancreatoduodenectomy for carcinoma of the ampulla of Vater. HPB (Oxford). 2007; 9:140–145. PMID: 18333130.

16. Park JS, Yoon DS, Kim KS, et al. Factors influencing recurrence after curative resection for ampulla of Vater carcinoma. J Surg Oncol. 2007; 95:286–290. PMID: 17326125.

17. Woo SM, Ryu JK, Lee SH, et al. Recurrence and prognostic factors of ampullary carcinoma after radical resection: comparison with distal extrahepatic cholangiocarcinoma. Ann Surg Oncol. 2007; 14:3195–3201. PMID: 17710498.

18. Westgaard A, Tafjord S, Farstad IN, et al. Pancreatobiliary versus intestinal histologic type of differentiation is an independent prognostic factor in resected periampullary adenocarcinoma. BMC Cancer. 2008; 8:170. PMID: 18547417.

19. de Paiva Haddad LB, Patzina RA, Penteado S, et al. Lymph node involvement and not the histophatologic subtype is correlated with outcome after resection of adenocarcinoma of the ampulla of Vater. J Gastrointest Surg. 2010; 14:719–728. PMID: 20107918.

20. Sierzega M, Nowak K, Kulig J, Matyja A, Nowak W, Popiela T. Lymph node involvement in ampullary cancer: the importance of the number, ratio, and location of metastatic nodes. J Surg Oncol. 2009; 100:19–24. PMID: 19384907.

21. Iacono C, Verlato G, Zamboni G, et al. Adenocarcinoma of the ampulla of Vater: T-stage, chromosome 17p allelic loss, and extended pancreaticoduodenectomy are relevant prognostic factors. J Gastrointest Surg. 2007; 11:578–588. PMID: 17468917.

22. Duffy JP, Hines OJ, Liu JH, et al. Improved survival for adenocarcinoma of the ampulla of Vater: fifty-five consecutive resections. Arch Surg. 2003; 138:941–948. PMID: 12963649.
23. Falconi M, Crippa S, Domínguez I, et al. Prognostic relevance of lymph node ratio and number of resected nodes after curative resection of ampulla of Vater carcinoma. Ann Surg Oncol. 2008; 15:3178–3186. PMID: 18712568.

24. Bogoevski D, Chayeb H, Cataldegirmen G, et al. Nodal microinvolvement in patients with carcinoma of the papilla of Vater receiving no adjuvant chemotherapy. J Gastrointest Surg. 2008; 12:1830–1837. PMID: 18791769.

25. Todoroki T, Koike N, Morishita Y, et al. Patterns and predictors of failure after curative resections of carcinoma of the ampulla of Vater. Ann Surg Oncol. 2003; 10:1176–1183. PMID: 14654474.

26. Nakao A, Harada A, Nonami T, et al. Prognosis of cancer of the duodenal papilla of Vater in relation to clinicopathological tumor extension. Hepatogastroenterology. 1994; 41:73–78. PMID: 8175122.
27. Dorandeu A, Raoul JL, Siriser F, et al. Carcinoma of the ampulla of Vater: prognostic factors after curative surgery: a series of 45 cases. Gut. 1997; 40:350–355. PMID: 9135524.

28. Terasawa H, Uchiyama K, Tani M, et al. Impact of lymph node metastasis on survival in patients with pathological T1 carcinoma of the ampulla of Vater. J Gastrointest Surg. 2006; 10:823–828. PMID: 16769538.

29. Furuse J, Takada T, Miyazaki M, et al. Guidelines for chemotherapy of biliary tract and ampullary carcinomas. J Hepatobiliary Pancreat Surg. 2008; 15:55–62. PMID: 18274844.

30. Komatsu S, Sonoyama T, Ochiai T, et al. Long-term complete response of multiple hepatic metastases from carcinoma of the papilla of Vater using intrahepatic infusion of 5-FU with low-dose cisplatin following pancreaticoduodenectomy. Int J Clin Oncol. 2008; 13:567–570. PMID: 19093189.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download