Abstract
Backgrounds/Aims
To investigate survival rates and prognostic factors of patients with gallbladder cancer who were treated with surgery and postoperative radiation therapy.
Methods
Seventeen gallbladder cancer patients who received surgery and postoperative radiotherapy from October 1989 to April 1998 were included in this retrospective study. Five patients had stage II, 8 patients had stage III, and 4 patients had stage IV disease according to the 1997 American Joint Committee on Cancer (AJCC) staging. All patients received ≥40 Gy of postoperative radiotherapy with a daily dose of 2.0 Gy/fraction and 15 patients received concurrent chemotherapy. An analysis was performed for the end-points of overall and disease-free survival.
Results
Of the 17 patients, 13 had no residual disease (R0), 1 had microscopic residual disease (R1), and 3 had macroscopic residual disease (R2) after surgery. Among patients with no residual disease, 4 had locoregional recurrences during the follow-up period. One patient with microscopic residual disease had local recurrence. The 5-year overall survival rate was 38.2%. The median overall survival time was 21 months and the median disease-free survival time was 12 months. Old age (≥60 years old), female gender, a high pathological stage (≥IVA), and the presence of residual disease after surgery were significant prognostic factors for disease-free survival.
Conclusions
Despite a high proportion of patients with advanced disease and macroscopic residual disease, the prognosis of gallbladder patients who had postoperative radiotherapy is encouraging. Additional investigation to improve the loco-regional control of gallbladder cancer patients with adverse prognostic factors is warranted.
Go to : 
Gallbladder cancer is a relatively rare disease; it comprised 1.2% of the total cancer incidence in Korea in the year 2007. It hardly generates any symptoms at early stages of the disease, and therefore the disease is often diagnosed at an advanced stage. Gallbladder cancer has a poor prognosis when it is diagnosed at an advanced stage with tumor invasion into the porta hepatis.
Complete surgical resection is the standard and only potentially curative treatment for early stage gallbladder cancer, but for advanced disease, a high recurrence rate remains the main problem after curative resection.12 It is still controversial if postoperative adjuvant therapy can improve the overall survival rate through reducing local recurrence and/or distant metastasis. In our institution, postoperative radiation therapy is given to gallbladder cancer patients with T2 or lymph node-positive disease.
The purpose of this study was to analyze the survival rates and prognostic factors of gallbladder cancer patients who received postoperative radiation therapy.
Go to : 
Seventeen gallbladder cancer patients who received more than 40 Gy of postoperative radiation therapy between October 1989 and April 1998 at Seoul National University Hospital and had more than 6 months of follow-up were included in this study.
All patients were diagnosed by preoperative abdominal ultrasonography or computed tomography. Percutaneous transhepatic biliary drainage or endoscopic nasobiliary drainage was done for patients with obstructive jaundice.
Four patients had simple cholecystectomy, 11 patients had extended cholecystectomy, and 2 patients had palliative resection. After surgery, 13 patients had no residual disease (R0), 1 had microscopic residual (R1), and 3 had gross residual disease (R2). Postoperative staging was done according to the 1997 TNM staging system.
Postoperative radiation therapy was initiated 4-8 weeks after surgery. A total of 40 Gy of radiation was delivered to the primary tumor bed and regional LN area using 2-field (anterior-posterior/posterior-anterior) or 3-field technique (anterior-posterior with lateral beams). Radiation therapy of 20 Gy (2.0 Gy/fraction, 5 fractions per week) was followed by 2 weeks of rest, and then another 20 Gy was administered. 5-fluorouricil (5-FU) 500 mg/m2 was administered intravenously for the first 3 days of each 2-week course of radiation therapy. Acute toxicities during concurrent chemoradiation therapy were graded according to the Common Toxicity Criteria version 2.0.
Survival was calculated from the date of surgery to the date of death or last surveillance. Disease-free survival was calculated from the date of surgery to the date of any type of relapse. The actuarial survival rates were calculated using the Kaplan-Meier method, and statistical significance was evaluated by the Log-rank test. Cox regression analysis was used to identify independent predictors of disease-free survival using factors found to be significant by univariate analysis.
Go to : 
Seventeen patients were retrospectively analyzed. Their characteristics are summarized in Table 1.
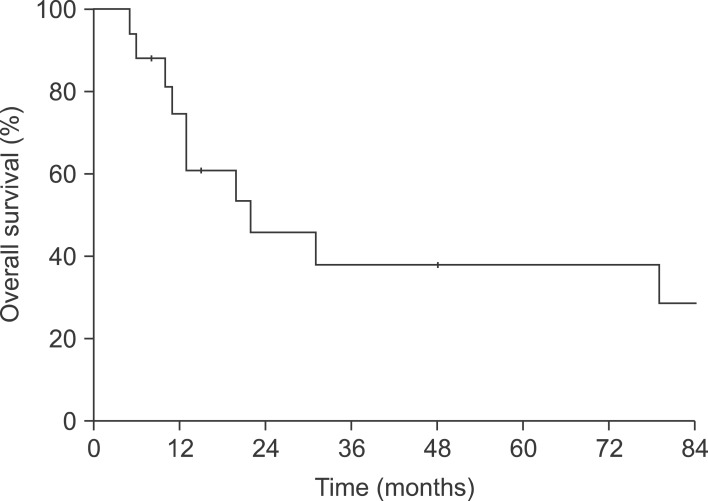
Median follow-up time was 15 months (range: 5-142 months). Median survival time for all patients was 21 months; the 5-year survival rate was 38.2% (Fig. 1). The five-year survival rate for the 14 patients without residual disease (R0) or with microscopic residual disease (R1) postoperatively was 43.3%.
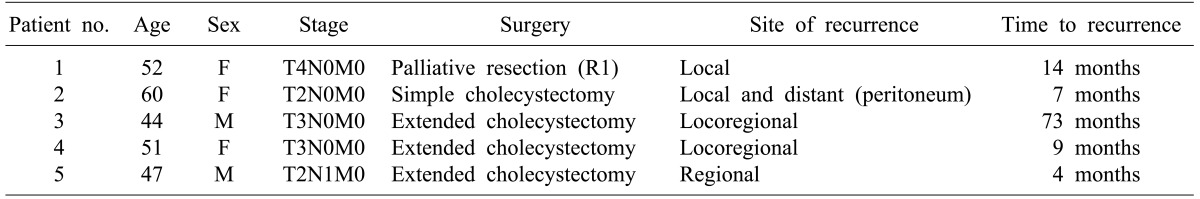
Among the 14 patients with R0 or R1 disease, 5 had recurrences (Table 2). One patient with R1 disease recurred at the site of the primary tumor bed 14 months after surgery. Two patients with R0 disease showed recurrence both at the primary tumor bed and regional lymph nodes. Another patient with R0 disease had both primary tumor bed recurrence and peritoneal seeding and still another had regional lymph node recurrence.
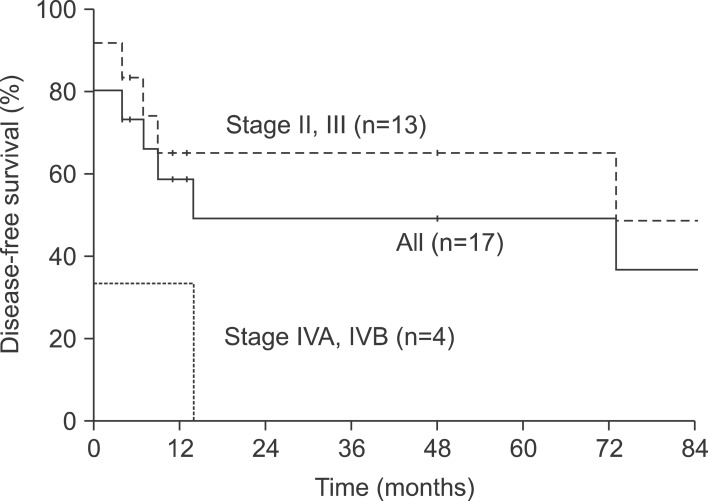
Median disease-free survival time for all patients was 12 months and 5-year disease-free survival rate was 48.9%.
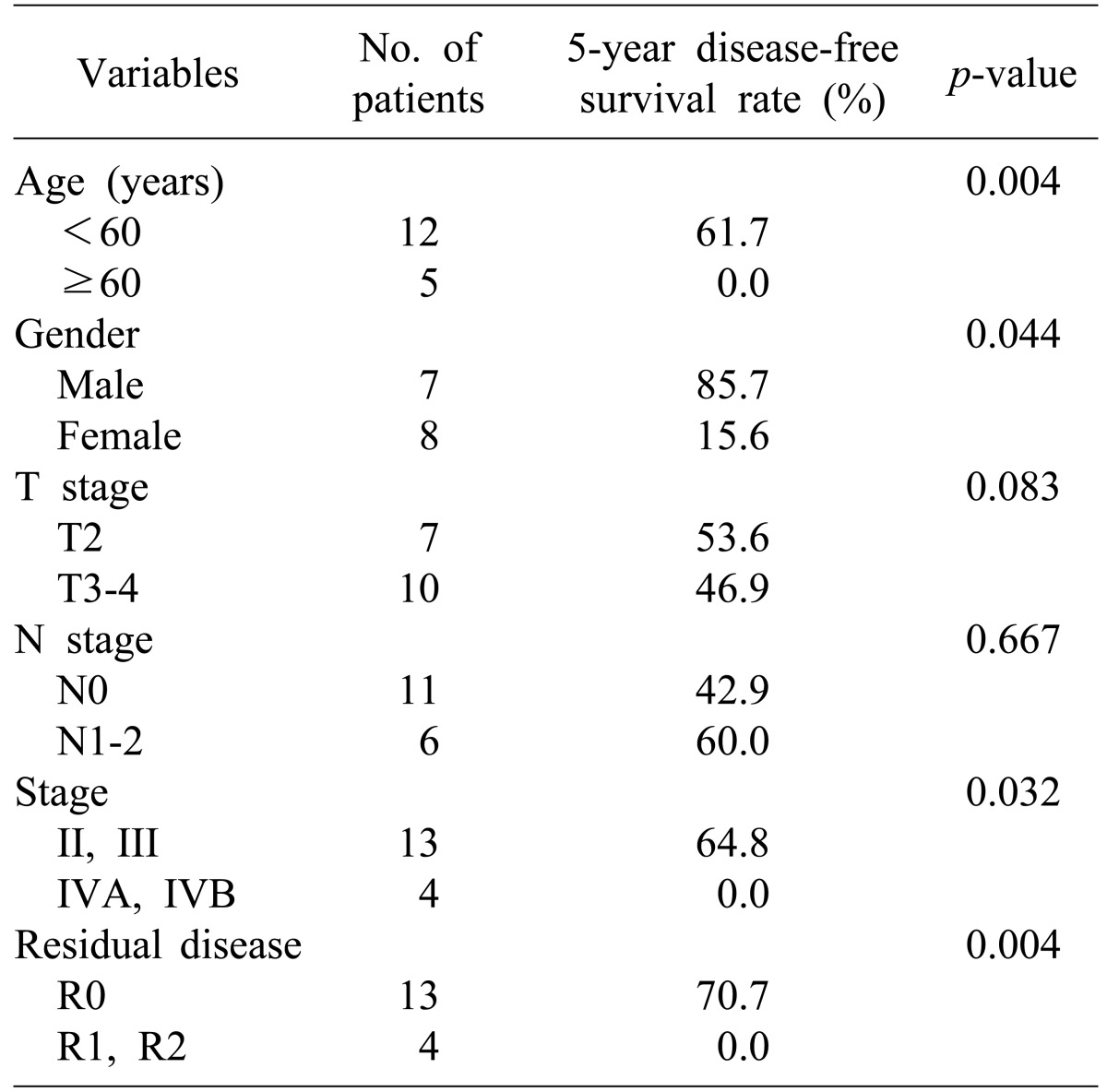
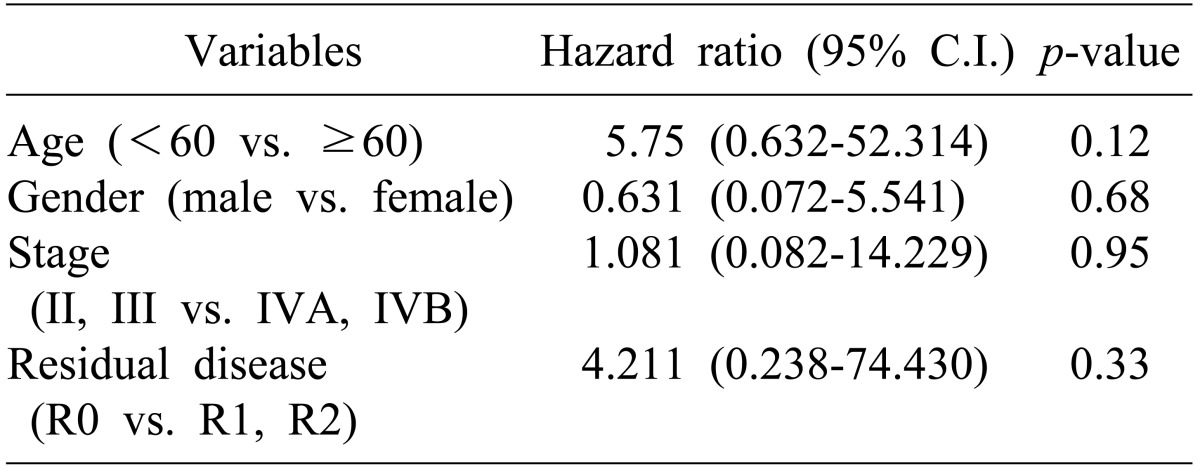
Univariate analysis was done to identify prognostic factors for disease-free survival of all patients. Old age (>60 years), female gender, advanced stage (≥IVA), and the presence of residual tumor after surgery (R1 or R2) were significant prognostic factors that adversely affected disease-free survival (Table 3, Fig. 2). There was no statistically significant prognostic factor in multivariate analysis (Table 4).
Seven patients (41% of the 17) had grade 2 hematological toxicities. As for non-hematological toxicities, 1 patient had grade 2 nausea and vomiting. No patient had severe (grade 3 or more) toxicity during postoperative chemoradiation therapy.
Maintenance chemotherapy after completion of chemoradiotherapy was used in 11 patients; 9 received 5-fluorouricil every four weeks (500 mg/m2/d, IV bolus for 5 days), and 2 received a combination of 5-fluorouracil (200 mg/m2/d), adriamycin (15 mg/m2/d), and mitomycin-C (5 mg/m2/d). Two patients experienced hematological toxicities that required chemotherapy dose reduction during maintenance chemotherapy.
Go to : 
In a recently published retrospective study of 73 gallbladder cancer patients who had radical resection, 5-year overall survival was 44.9%.3 None of the patients had postoperative residual disease and 60% of them had T1-T2N0M0 disease. In the present study, 5-year overall survival was 38.2% despite the fact that 60% of the study population had T3-T4 disease, more than one third had lymph node metastasis, and 18% (3/17) had gross residual disease. Excluding patients with gross residual disease, 5-year overall survival was 43.3%. Therefore, the treatment outcome at our institution was comparable to other reports.456 But it would be too hasty to conclude that there was any benefit of postoperative chemoradiotherapy for gallbladder cancer patients as there was no control (surgery only) group in current study.
Kim et al.7 reported recurrence patterns and risk factors influencing recurrence of gallbladder cancer patients who had radical resection. Seventy-two of 166 patients (43%) had recurrence events that were classified as local, regional and distant disease recurrence. Regional lymph node recurrence (20/166, 28%) was observed most frequently, followed by intrahepatic metastasis (16/166, 22%). They also reported that regional lymph node metastasis was an independent predictor of tumor recurrence by multivariate analysis. In the present study, although the number of cases was very small, regional recurrence was most frequently observed in patients who had disease recurrence after extended cholecystectomy. Based on this finding, regional lymph node recurrence was still the major pattern of recurrence for gallbladder cancer patients who had postoperative radiation therapy after curative resection.
It is still controversial whether postoperative radiation therapy reduces local recurrence and increases survival. Because of the low incidence of gallbladder cancer, most published reports have tried to identify the efficacy of adjuvant therapy by retrospective analysis and their study populations have had variable characteristics. Jarnagin et al.8 compared the pattern of failure of hilar cholangiocarcinoma with that of gallbladder carcinoma and reported that gallbladder cancer more frequently recurs at a distant site than a locoregional site in contrast with hilar cholangiocarcinoma. Based on their findings, they questioned the rationale of postoperative radiation therapy for gallbladder cancer patients. In the study by Kim et al.,7 40 of 166 patients received postoperative adjuvant therapy, but they did not have any gain in disease-free survival. But in another study, postoperative chemoradiotherapy was a statistically significant prognostic factor in addition to T, N stage and histology for stage I or II gallbladder cancer patients.3 Recently, Korean researchers reported that postoperative chemoradiation therapy was an independent prognostic factor for disease-free survival of gallbladder cancer patients with lymph node-positive T2/T3 disease.9
The small number of subjects in the study population is a limitation of this study. In univariate analysis, age (≥60 vs. <60), gender (female vs. male), stage (IVA and IVB vs. II and III), and postoperative residual disease (R1 and R2 vs. R0) were statistically significant variables for disease-free survival. But none of these were statistically significant prognostic factors in multivariate analysis.
In conclusion, treatment outcomes for gallbladder cancer patients who underwent postoperative chemoradiotherapy is comparable to those of other reports even though our study population had prognostic factors that predicted relatively poor outcomes. A multi-institutional prospective study is needed to validate the role of postoperative chemoradiotherapy for gallbladder cancer patients. In addition, greater effort to improve locoregional control by increasing radiation dose or by introducing a new chemotherapy regimen is required, because regional lymph node recurrence is still the main pattern of failure for gallbladder cancer patients after postoperative chemoradiation therapy.
Go to : 
References
1. Duffy A, Capanu M, Abou-Alfa GK, et al. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC). J Surg Oncol. 2008; 98:485–489. PMID: 18802958.

2. Mahantshetty UM, Palled SR, Engineer R, Homkar G, Shrivastava SK, Shukla PJ. Adjuvant radiation therapy in gall bladder cancers: 10 years experience at Tata Memorial Hospital. J Cancer Res Ther. 2006; 2:52–56. PMID: 17998675.

3. Gold DG, Miller RC, Haddock MG, et al. Adjuvant therapy for gallbladder carcinoma: the Mayo Clinic Experience. Int J Radiat Oncol Biol Phys. 2009; 75:150–155. PMID: 19297105.

4. Park JI, Kim JS, Kim KH, et al. Analysis of prognostic factors affecting survival in patients with gallbladder cancer. Korean J Hepatobiliary Pancreat Surg. 2010; 14:173–183.
5. Czito BG, Hurwitz HI, Clough RW, et al. Adjuvant external-beam radiotherapy with concurrent chemotherapy after resection of primary gallbladder carcinoma: a 23-year experience. Int J Radiat Oncol Biol Phys. 2005; 62:1030–1034. PMID: 15990005.

6. Kresl JJ, Schild SE, Henning GT, et al. Adjuvant external beam radiation therapy with concurrent chemotherapy in the management of gallbladder carcinoma. Int J Radiat Oncol Biol Phys. 2002; 52:167–175. PMID: 11777635.

7. Kim WS, Choi DW, You DD, Ho CY, Heo JS, Choi SH. Risk factors influencing recurrence, patterns of recurrence, and the efficacy of adjuvant therapy after radical resection for gallbladder carcinoma. J Gastrointest Surg. 2010; 14:679–687. PMID: 20094817.

8. Jarnagin WR, Ruo L, Little SA, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer. 2003; 98:1689–1700. PMID: 14534886.
9. Cho SY, Kim SH, Park SJ, et al. Adjuvant chemoradiation therapy in gallbladder cancer. J Surg Oncol. 2010; 102:87–93. PMID: 20578085.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download