This article has been
cited by other articles in ScienceCentral.
Abstract
Backgrounds/Aims
To find independent predictors that affect the survival in patients with hepatic metastasis of colorectal cancer after surgery and to devise a risk scoring system.
Methods
Among 150 patients who underwent hepatic resection after diagnosis of colorectal cancer with hepatic metastasis between March 1994 and February 2009, we analyzed clinical, pathologic and outcome data retrospectively.
Results
The 1-year survival rate was 83%, and the 5-year survival rate was 35%. Nine factors were found to be independent predictors of adverse outcome by univariate analysis: stage of primary tumor, CA19-9 >36 U/ml, extrahepatic disease, distribution of the hepatic tumor, number of hepatic tumors >3, largest hepatic tumor >5 cm, total size >10 cm, CEA >10 ng/ml, and metachronous cancer. The last two of these criteria were also significant risk factors on multivariate analysis. When these criteria were used as a risk scoring system, assigning one point for each criterion and dividing the cases into A, B and C groups, the total score was highly predictive of outcomes (p<0.001). No patients in group C (6 to 9 points) were long-term survivors.
Conclusions
Long-term outcome can be predicted from nine criteria that are readily available for all patients. Patients meeting up to two criteria (group A) are more likely to have a favorable outcome compared to the three or over (groups B and C). This scoring system may offer an easy, rapid, and reliable prognostic indicator of survival outcome after hepatic resection in patients with hepatic metastasis from colorectal cancer.
Go to :

Keywords: Colorectal cancer, Hepatic metastasis, Hepatic resection, Clinical risk scoring system
INTRODUCTION
Colorectal cancer is one of the most common cancers in Korea as well as in western countries and its incidence is increasing. Major organs of distant metastases from colorectal cancer are liver, lung, and bone.
1 There is no accurate report on the incidence of hepatic metastases in our country, but the main cause of death from colorectal cancer is associated with hepatic metastases.
2 In the North America, the incidence of hepatic metastases has been reported as 15-25% in the synchronous, 25-50% in the metachronous.
345
Hepatic resection has been regarded as a safe and effective treatment option in patients with hepatic metastasis from colorectal cancer. Hepatic resection can be accomplished with an associated surgical mortality rate uniformly less than 5%, even for resection of as much as 80% of the liver.
6789 In general, the median survival period for untreated patients with hepatic metastases from colorectal cancer is 6-12 months and 12-18 months for patients treated with chemotherapy alone. On the other hand, complete resection of detected hepatic metastases results in a 5-year survival for one third of patients even in the era that current adjuvant chemotherapy had not been established. A recent series reported a nearly 60% 5-year survival rate when combined with modern neoadjuvant and adjuvant chemotherapy and other ablation therapies.
1011
Many investigators have identified clinical variables that predict long-term survival after hepatic resection in patients with hepatic metastases from colorectal cancer. Factors commonly associated with adverse outcomes have been primary tumor stage, regional nodal positivity, synchronous presentation of metastatic tumors, disease-free survival, number of metastatic tumors, bilateral tumors, size of the tumor, carcinoembryonic antigen (CEA) level on the diagnosis of hepatic metastasis, positive resection margins, extrahepatic disease.
121314 Several investigators have attempted to develop a scoring system using these clinical variables. But such clinical scoring systems have not been widely used.
In this study, we attempted to make a useful clinical risk scoring system using clinical and pathologic variables for predicting recurrence after hepatic resection for colorectal cancer with hepatic metastasis.
Go to :

METHODS
Of the 402 patients who underwent hepatectomy for hepatic metastases from colorectal cancer between March 1994 and February 2009 at the department of surgery, Chonbuk National University Hospital, 150 were selected for the study. We excluded the 252 patients who did not receive surgery for a primary cancer lesion, who had multiorgan distant metastasis, who died due to surgical complications, who had a history of cancer at another site, or who had an insufficient medical record.
We studied 14 factors as followed: gender, age, primary tumor location, primary tumor stage, primary tumor pathologic grade, regional nodal positivity, number of metastatic lesions, distribution of metastatic lesions, tumor size of the largest hepatic metastasis, total size of hepatic metastases, CEA and CA19-9 levels, temporal relationship, and extrahepatic lesions.
Univariate analysis, multivariate analysis, Kaplan-Meier method, and Cox proportional hazard regression analysis were performed using SPSS (ver. 17).
Go to :

RESULTS
Mean age at the time of diagnosis was 60.9 years (range: 27-84) and 105 (70.0%) were men. Median follow-up was 27.2 months. The rectum was the most common primary tumor site (n=81; 54.0%). The most common initial primary tumor stage was stage IV (n=89; 59.3%), and there were 2 patients with stage I (
Table 1).
Table 1
Primary tumor stage of patients with colorectal cancer

The overall survival rate was 83% at 1 year after hepatic resection and 35.9% at 5 years. All patients with stage I colorectal cancer showed 100% survival at 1, 3 and 5 years. Survival for patients with stage II was 100% at 1 year, 79.6% at 3 years, and 42.6% at 5 years; for patients with stage III disease, survival was 93.8% at 1 year, 71.8% at 3 years, and 57.1% at 5 years; for stage IV, it was 72.7% at 1 year, 46.3% at 3 years, and 29.4% at 5 years (
p<0.001) (
Fig. 1).
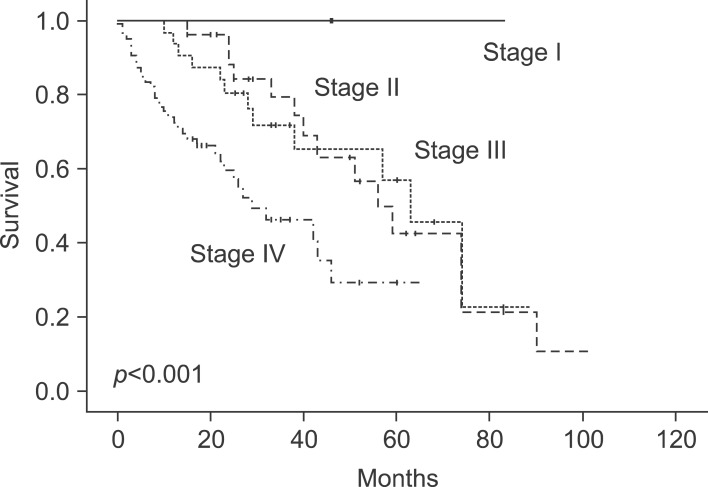 | Fig. 1Cumulative survival curves of the groups as related to the stages. 1-, 3-, and 5-year survival rate was all 100% in stage I (n=2). 1-, 3-, and 5-year survival rate was 100%, 79.6%, and 42.6% each in stage II (n=27). 1-, 3-, and 5-year survival rate was 93.8%, 71.8%, and 57.1% each in stage III (n=32). 1-, 3-, and 5-year survival rate was 72.7%, 46.3%, and 29.4% each in stage IV (n=89) (p<0.001).
|
Hepatic metastases discovered within 12 months after primary colorectal resection was defined as a synchronous metastasis; after 12 months, it was defined as metachronous. Comparison of survival between synchronous and metachronous metastatic tumors were significant: 78.8% at 1 year, 49.4% at 3 years, and 23.3% at 5 years for synchronous tumors, and 100% at 1 year, 96% at 3 years, and 77.5% at 5 years for metachronous tumors (
Fig. 2).
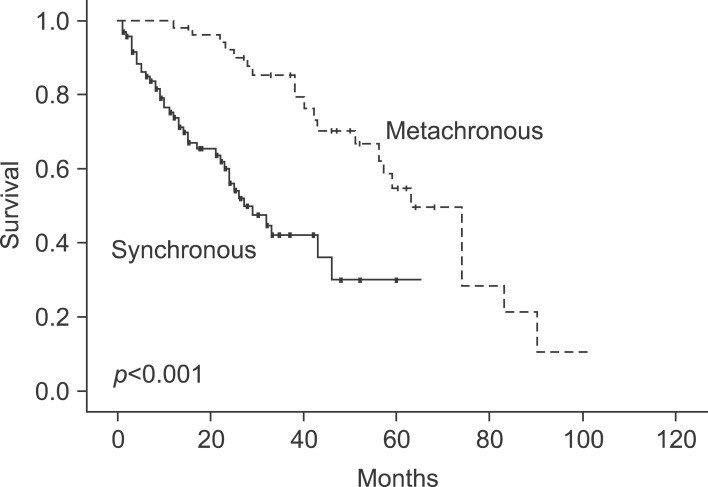 | Fig. 2Cumulative survival curves of the groups between synchronous and metachronous metastasis. The 1-, 3-, and 5-year survival rate was 78.8%, 49.4%, and 23.3% each in synchronous (n=122) and 1-, 3-, and 5-year survival rate was 100%, 96%, and 77.5% each in metachronous (n=28), respectively (p<0.001).
|
Gender, age, primary tumor grade, and primary tumor location were not significant in univariate analysis. Primary tumor stage (stage IV), CEA level on the diagnosis of hepatic metastasis (>10 ng/ml), CA19-9 level on of hepatic metastasis (>36 U/ml), synchronous metastasis, extrahepatic metastasis, bilobar hepatic metastasis, number of metastatic lesion (≥3), the largest hepatic metastasis tumor size (>5 cm), and the total size of hepatic metastases (>10 cm) were associated with significantly poorer prognosis in univariate analysis (
Table 2). Among the factors that predicted poor outcomes, synchronous metastasis and CEA level on diagnosis of hepatic metastasis (>10 ng/ml) were significant in both univariate and multivariate analyses (
Table 3).
Table 2
Significant prognostic factors for survival in patients with hepatic metastasis after primary colorectal resection by univariate analysis
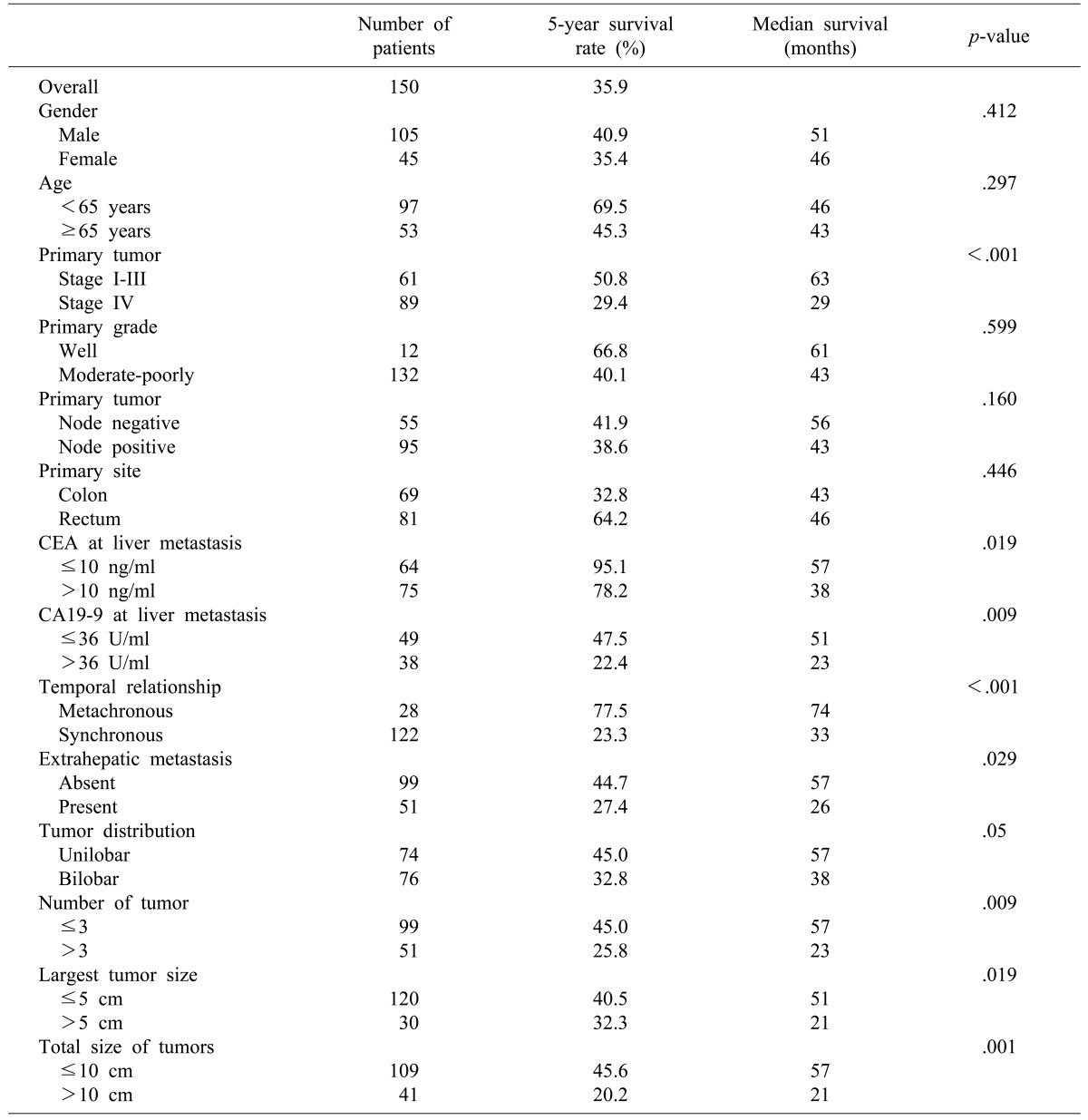

Table 3
Significant prognostic factors for survival in patients with hepatic metastasis after primary colorectal resection by multivariate analysis


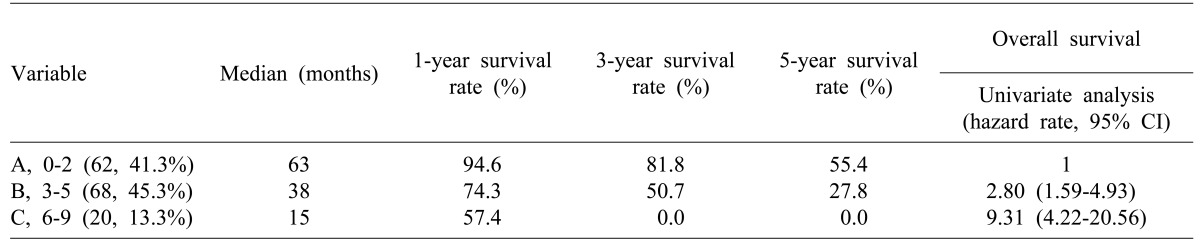
By assigning 1 point for each of the 9 significant factors in univariate analysis and adding the scores yielded total scores from 0 to 9 points. Using this scoring system, we divided patients into three groups: Scores of 0 to 2 were group A, 3 to 5 were group B, and 6 to 9 were group C. Groups were compared for long-term survival rates. In the case of group C, median survival was 15 months and the survival rate was 57.4% at 1 year after hepatic resection; there were no patients with long-term survival over 3 years (
Table 4).
Table 4
Comparison of survival rates for groups related to clinical risk scoring system

Go to :

DISCUSSION
The liver is the first major organ reached by venous blood draining from the gastrointestinal tract. Cancer cells traveling by hematogenous spread, therefore, have a high likelihood of arriving and lodging within the liver. This would explain the observation that the liver is the most common organ of distant metastases from colorectal cancer.
15
In the past, hepatic resection was regarded with great skepticism.
16 However, the evolution of liver surgery uniformly lowered the mortality rate and made possible extensive hepatic resection. Until recently, according to many reports on patients with liver metastasis of colorectal cancer, hepatic resection has been established as a safe and effective treatment that leads to a relatively high long-term survival.
171819
Many studies have reported clinical risk scoring systems that consist of clinical and pathologic factors and are helpful for predicting long term survival (
Table 5). Gayowski et al.
18 included size and distribution of tumors and proposed a staging system for hepatic colorectal metastases based on the size of liver metastases (>2 cm), number of lesions (>1), and bilobar distribution of tumors. Cady et al.
20 proposed a scoring index based on four risk factors: disease-free interval after treatment of the primary tumor, number of liver tumors (>4), CEA level, and margin of resection. Nordlinger et al.
21 attempted to incorporate additional prognostic criteria for outcomes and proposed a system based on seven criteria: age >60 years, stage of the primary tumor, >4 liver metastases, synchronous disease, size of the largest lesion >5 cm, CEA level >30 ng/ml, and positive margins.
Table 5
Predictors of recurrence after hepatic resection for metastatic colorectal cancer
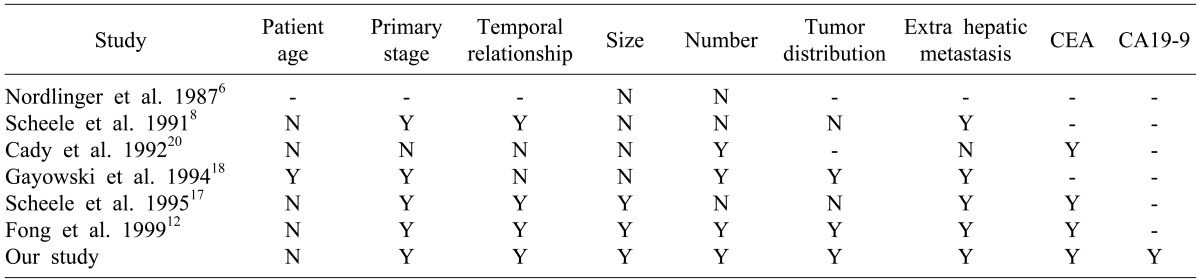

Fong et al.
12 found seven criteria to be independent prognostic factors for outcomes. However, except for the presence of extrahepatic disease and a positive surgical margin, their other five criteria-nodal status of the primary tumor, disease-free interval from diagnosis of the primary tumor to discovery of the liver metastases (<12 months), number of tumors (>1), preoperative CEA level >200 ng/ml, and size of the largest tumor >5 cm - were incorporated into a proposed clinical risk scoring system.
Our risk scoring system included the clinical and pathological factors listed above, and other factors (CA19-9, primary tumor grade). As well using our scoring system, group A had good prognosis compared with the other groups. Survival in group A was 94% at 1 year, 81% at 3 years, and 55% at 5 years. Furthermore, 5-year survival rate in group C, which scored over 6 points, was 0%. Therefore, if the score after surgery is over 3 points (groups B and C group, a more aggressive chemotherapy is necessary.
Due to recent developments in molecular genetic analysis, more sophisticated markers, such as tumor ploidy, oncogene expression, or tumor suppressor gene expression, may further stratify patients with regard to outcome.
2021 However, such molecular or genetic markers are not likely to be readily available except at a few academic centers; this limits their usefulness and also dooms any system incorporating their use in terms of wide applicability.
In conclusion, CEA >10 ng/ml and metachronous metastases had significance in both univariate and multivariate analyses among nine criteria. All nine criteria were used in our risk scoring system. We assigned one point for each criterion and divided all cases into three groups. The risk scoring system devised in this study for patients with hepatic metastases from colorectal cancer is relatively simple and may enable us to accurately predict the outcomes.
Go to :

ACKNOWLEDGEMENTS
This study was support by a grant of the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (0620220).
Go to :

References
1. Lee WK, Kim SB, Cho EH, Hwang DY, Moon SM. Outcomes of a hepatic resection for colorectal-carcinoma liver metastases. J Korean Soc Coloproctol. 2010; 26:204–210.

2. Choi PW, Kim HC, Jung SH, et al. Outcomes after a hepatic resection for multiple hepatic metastases from colorectal cancer. J Korean Soc Coloproctol. 2008; 24:100–106.

3. Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990; 77:1241–1246. PMID:
2253003.

4. Finlay IG, McArdle CS. Occult hepatic metastases in colorectal carcinoma. Br J Surg. 1986; 73:732–735. PMID:
3756437.

5. Li Destri G, Di Cataldo A, Puleo S. Colorectal cancer follow-up: useful or useless? Surg Oncol. 2006; 15:1–12. PMID:
16891116.

6. Nordlinger B, Quilichini MA, Parc R, Hannoun L, Delva E, Huguet C. Hepatic resection for colorectal liver metastases. Influence on survival of preoperative factors and surgery for recurrences in 80 patients. Ann Surg. 1987; 205:256–263. PMID:
3827361.
7. Schlag P, Hohenberger P, Herfarth C. Resection of liver metastases in colorectal cancer--competitive analysis of treatment results in synchronous versus metachronous metastases. Eur J Surg Oncol. 1990; 16:360–365. PMID:
2379594.
8. Scheele J, Stangl R, Altendorf-Hofmann A, Gall FP. Indicators of prognosis after hepatic resection for colorectal secondaries. Surgery. 1991; 110:13–29. PMID:
1866690.
9. Iwatsuki S, Shaw BW Jr, Starzl TE. Experience with 150 liver resections. Ann Surg. 1983; 197:247–253. PMID:
6830332.

10. Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002; 235:759–766. PMID:
12035031.

11. Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004; 239:818–825. PMID:
15166961.

12. Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999; 230:309–318. PMID:
10493478.
13. Charnsangavej C, Clary B, Fong Y, Grothey A, Pawlik TM, Choti MA. Selection of patients for resection of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006; 13:1261–1268. PMID:
16947009.

14. Fahy BN, D'Angelica M, DeMatteo RP, et al. Synchronous hepatic metastases from colon cancer: changing treatment strategies and results of surgical intervention. Ann Surg Oncol. 2009; 16:361–370. PMID:
19050976.

15. Ruiz-casado A, Pereira F. Liver metastases of colon cancer: New therapeutic approaches, Neoadjuvant chemotherapy. Oncologia. 2006; 29:3–15.

16. Silen W. Hepatic resection for metastases from colorectal carcinoma is of dubious value. Arch Surg. 1989; 124:1021–1022. PMID:
2673139.

17. Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995; 19:59–71. PMID:
7740812.

18. Gayowski TJ, Iwatsuki S, Madariaga JR, et al. Experience in hepatic resection for metastatic colorectal cancer: analysis of clinical and pathologic risk factors. Surgery. 1994; 116:703–710. PMID:
7940169.
19. Rosen CB, Nagorney DM, Taswell HF, et al. Perioperative blood transfusion and determinants of survival after liver resection for metastatic colorectal carcinoma. Ann Surg. 1992; 216:493–504. PMID:
1417198.

20. Cady B, Stone MD, McDermott WV Jr, et al. Technical and biological factors in disease-free survival after hepatic resection for colorectal cancer metastases. Arch Surg. 1992; 127:561–568. PMID:
1575626.

21. Nordlinger B, Guiguet M, Vaillant JC, et al. Association Française de Chirurgie. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Cancer. 1996; 77:1254–1262. PMID:
8608500.

Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download