Abstract
Purpose
This study is designed to ascertain the most effective quantity and injection route of hepatocytes in an acute liver failure model induced by massive liver resection in rats.
Methods
Rats weighing 450 to 650 gm underwent partial hepatectomy that was 80% of their liver weight, resulting in acute liver failure. Hepatocytes were obtained by perfusing collagenase (Wako, Japan) solution through portal vein into liver of the allogenic rat. These hepatocytes were injected into different places with different dosage. The experimental groups were divided into the Control group, Splenic group I (2×106 cells into splenic capsule), Splenic group II (2×107 cells into splenic capsule), Portal vein group (2×107 cells into portal vein), Subperitoneal group (2×107 cells into subperitoneum). The experimental animals were observed carefully for 5 days for assessment of survival and regeneration of liver. Liver function tests including serum alanine aminotransferase (ALT), total bilirubin, gamma-glutamyl transferase (γ-GTP) on postoperative 1, 2, 3, 5th days and histologic examinations of specimens obtained from each respective groups on postoperative 5th day were performed.
Results
Serum ALT level on postoperative day 1 peaked and then gradually normalized showing statistical significance (p=0.035). Study groups showing statistically significant difference under repeated anova analysis were between the Splenic group II and Control (p=0.035), and between the Splenic group II and Portal vein group (p=0.001) with respect to serum ALT levels. Also, progression of each study group showed statistical significance. (p=0.02). Serum total bilirubin and r-GTP did not show any significant difference.
Acute liver failure is experienced commonly in clinical practice. The causes are diverse such as drug intoxication, viral diseases, massive liver resection, etc. Although various treatment procedures have been developed, it shows a 40~80% high mortality rate. Nonetheless, once liver failure is recovered, it is a disease without sequelae.123 Until now, efforts have been made to recover liver function by applying various treatment methods in acute liver failure, however the results are equivocal. Only liver transplantation has been accepted as the most definitive treatment method.13 Nonetheless, problems of liver transplantation include that the there are insufficient donors, mortality rates and complications result due to prolonged surgery, and the long term use of immunosuppressants induces side effects. Presently, symptomatic treatment methods have developed substantially, and thus the major trends are conservative treatment, hemodialysis, and hemofiltration. Nevertheless, treatment outcomes are not satisfactory, and thus as an alternative treatment bioartificial liver supports have been used. Bioartificial liver supports have been investigated as a middle stage until liver transplantation can be performed in acute liver failure patients, or as a means for prolongation in patients in whom liver transplantation is not a feasible option, or in patients with failed liver transplantation. Therefore, bioartificial liver support has not received enthusiastic as a clinical application.45 As an substitute, hepatocyte transplantation has emerged as an alternative treatment modality, as it has advantages such as a role as a temporary metabolic adjuvant therapy until hepatocytes regenerate while preserving the original liver in acute liver failure patients, and it allows patients to gain time while waiting for liver transplantation due to lack of donor livers, or patients waiting for re-transplantation because of acute or chronic rejection after liver transplantation. 67 It is thought that liver cell transplantation is favorable treatment method that complements the shortcomings of symptomatic treatments and liver transplantation, however, it is still in the early stages. In 1997, Strom et al.7 reported that in acute liver failure patients, survival rates were increased by transplanting human hepatocytes and subsequently liver cell transplantation has been attempted in clinical practice, but this procedure still suffers from numerous problems that have to be resolved such as isolation of a sufficient amount of hepatocytes, appropriate time for follow-ups, and immune suppression methods.6
Therefore, the authors examined isolated liver cells and injected various numbers of liver cells via diverse routes into syngeneic white rats with induced acute liver failure by massive liver resection. In order to ascertain the most effective and optimal number of hepatocytes and injection route, and to apply this concept to liver failure patients in the future.
Sprague-Dawley male rats weighing 450~650 gm were used. A one week period was allowed prior to the experiment for adjustment to laboratory environment. In the animals facility lights were turned on and off every 12 hours repeatedly, ambient temperature was maintained at 25℃ and humidity was maintained at 40%. After surgery, the rats were permitted to freely feed 5% glucose solution, and immunosuppressants were not used. All experiments were performed after obtaining approval from the Catholic Medical Center Ethics Committee for animal experiments and under their supervision the animal experiment guidelines were followed.
Acute liver failure was induced by the modified method of Higgins and Anderson.8 Experimental rats were subject to 0.3 µ/10 gm Zolazepam (Zoletil®, Virbac, Carros cedex, France) intraperitoneal injection in order to induce general anesthesia, and the liver was exposed by a median incision. The vascular structures of the middle lobe, left anterior lobe, and right inferior lobe of the liver was ligated with black silk 3-0, and the corresponding liver was resected. Excluding the right upper lobe and the omental lobe, 80% of the liver was resected (Fig. 1). After surgery, hepatocytes were injected according to each respective study group, and to supplement body fluids approximately 2 ml saline was injected through the penile vein.
In an identical manner, general anesthesia was induced by injecting anesthetics into the peritoneal cavity of experimental rats, and subsequently the liver was exposed by a median incision, a 22 gauge intravenous needle was catheterized into the portal vein 5 mm inferior to the liver, immobilized, and perfused with perfusion solution that was prepared in advance. The perfusion solution consisting of 300 ml HEPES buffer was injected at a rate of 20 ml per minute, and after the 2-stage perfusion method upon completion of perfusion the liver parenchyma was dissolved by injecting 15 ml collagenase solution (collagenase type IV, Wako, Tokyo, Japan) through the same route at a rate of 15 ml per minute was used (Fig. 2).
Thereafter, the liver was resected, washed, the liver capsule was resected and spread in HBSS solution. Other cells, connective tissues and contaminants were removed by centrifuging 3 times (50 g, 300 seconds), and collagenase solution was applied to obtain hepatocytes. Trypan blue exclusion test was performed to calculate the number of viable hepatocytes and the liver cells were prepared for transplantation (Fig. 3).
Experiment groups were classified according to number of injected viable hepatocytes, and the injection routes are as follows:
Without liver cell transplantation, only culture medium was injected into the subcapsular portion of spleen.
2×106/ml hepatocytes in 1 cc were injected into the subcapsular portion of spleen.
2×107/ml hepatocytes in 1 cc were injected into the subcapsular portion of spleen.
2×107/ml hepatocytes in 1 cc were injected to the subperitoneal area in the right upper abdomen to rats in which 80% of the liver was resected.
Cells for transplantation that were prepared in advance according to the cell number were injected to the corresponding group using a 25 gauge needle which was connected to a 1 ml syringe. Subsequently, to prevent cell loss and hemorrhage, the injection area was compressed with a fabric for 5 minutes. After confirming hemostasis, the abdominal wall was sutured, and to supplement the loss of fluid that occurred during liver resection, 2 ml saline was injected into the penile vein, and antibiotics (gentamicin, 1.25 µl/10 g) and an analgesic (ketoprofen, 10 µl/10 g) were injected into both tigh. At that time, to minimize the deterioration of the hepatocyte vitality level, transplantation to rats that received liver resection was performed immediately after hepatocyte isolation, and therefore, the dates of hepatocyte separation and transplantation of each experiment group was different.
The outcomes and survival of rats that received liver cell transplantation were observed for 5 days after surgery. The weight of the rats and the weight of the resected liver on the day of surgery, and the weight of rats and the weight of the resected liver of rats sacrificed 5 days after surgery were examined.
Blood was collected from the ophthalmic plexus 1 day, 2 days and 3 days after surgery. On day 5, blood was collected while sacrificing rats that received transplantation, centrifuged, and the serum was stored in a freezer. Using the stored serum, serum alanine aminotransferase (ALT), and γ-glutamyl transferase (γ-GTP) were measured by enzymatic methods. Total bilirubin was measured by a colorimetric method. In regard to syngeneic white rats, the standard values of the blood tests are almost identical, and it was directly associated with mortality rate after surgery, and thus blood tests were not performed prior to surgery.
On day 5 after surgery, experimental animals were sacrificed, and the liver was extracted. According to the experiment groups, the spleen, liver, and subperitoneal tissues were obtained, fixed in formalin, stained with Hematoxylin-Eosin, and morphologically examined for distribution of hepatocytes, survival, etc.
Experimental values were presented as a mean±standard deviation. The Student t-test or the Mann-Whitney test was used for comparison of continuous variables according to their distribution. For comparison of categorical variables, chi-square or Fisher's extract test was used. SPSS 17.0 (SPSS, Inc., Chicago, ILL, USA) was used for performance of all statistical analysis. p<0.05 was considered significant. Comparative analysis was performed by repeated ANOVA. p<0.05 was defined as statistically significant.
In the total 5 experiment groups, 3 rats died after surgery. One rat in the spleen group and 1 rat in the subperitoneal group died 1 day after surgery. One rat in the portal vein group died 2 days after surgery. The remaining rats survived until sacrifice at 5 days after surgery.
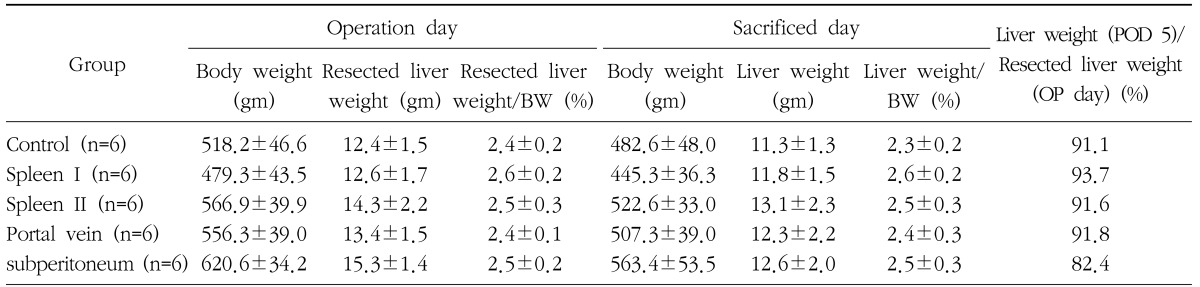
The mean pre-operative weight was 548.2±61.5 gm and the mean weight of the resected liver was 13.6±0.03 gm. The mean weight of the liver resected during surgery was 2.5±0.2%, the mean weight of liver at the time of sacrifice was 12.6±2.0 gm, and the mean ratio of liver weight to body weight was 2.5±0.26%. During the 5 day observation period, body weight was reduced to approximately 90.1~93%. Liver regeneration on day 5 after liver resection showed that ratio of liver weight to body weight recovered to the resected weight, and the regeneration rate between the groups was not significantly different (Table 1).
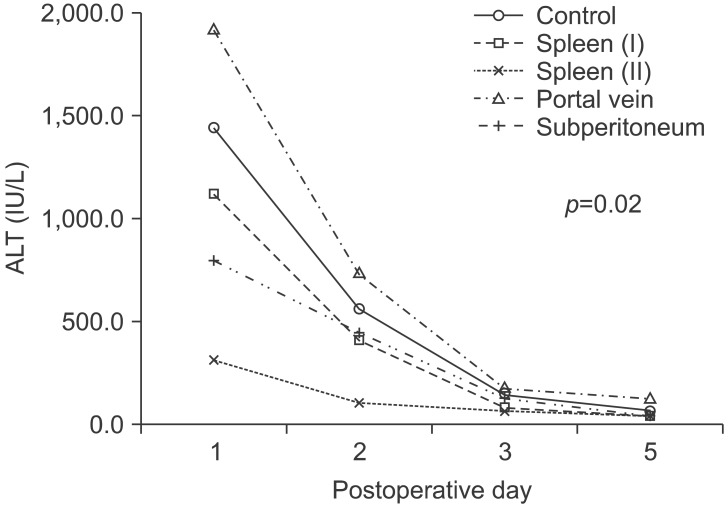
In each group, blood was collected 1 day, 2 days, 3 days, and 5 days after surgery, and ALT, total bilirubin, and r-GTP were measured. We observed a trend of ALT maximal increase in each group 1 day after surgery, and with time the value gradually normalized that was statistically significant (p=0.035). One day after surgery ALT values in the Spleen group II was lowest (312.5±93.9 IU/L), and which normalized 2 days after surgery. On the other hand, ALT values were elevated substantially (average 1,913.2±811.9 IU/L) 1 day after surgery in the Portal vein group, while 5 days after surgery it was 119.6±159.6 IU/L, and slightly elevated values were maintained which was different from other groups. One day after surgery, ALT values of the Spleen group II was 312.5±93.9 IU/L, and it was significantly lower than the 1,442.3±377.0 IU/L of the Control group (p=0.094). ALT values in the Portal vein group was 1,913.2±811.9 IU/L, which was higher than the Control group, but it was not statistically significant (p=0.157). One day after surgery, ALT values of the Spleen group I were 1,119.6±234.0 IU/L, and in the Subperitoneal group it was 794.4±141.2 IU/L. Although not statistically significant, the level was lower than the Control group. Two days after surgery, the Spleen group II was 102.0±35.0 IU/L, and it was lower than the 558.7±330.2 IU/L of the control group, but without statistical significant (p=0.282). Other groups also did not show significant differences from the Control group. Three and five days after surgery, the value of the all groups showed lower values, and marked differences were not observed. Linear comparison of each separate group that showed statistically significant difference were the Spleen group II and the Control group (p=0.017), as well as the Portal vein group (p=0.001). The pattern of the change in all groups also showed statistically significant difference (p=0.02) (Fig. 4).
The total bilirubin values 1 day after surgery in the Control group was 3.8±3.3 mg/dl, which was the highest level, in the Portal vein group it was 3.6±2.3 mg/dl, the Subperitoneal group was 2.6±0.9 mg/dl, the Spleen group II was 2.3±1.2 mg/dl, the Spleen group I was lowest value of 2.0±1.2 mg/dl, all without statistical significance. In all groups except the Subperitoneal group, a total bilirubin showed decreasing trend with time, but no statistical difference was seen between the groups. In the Subperitoneal group, blood bilirubin values increased with time and maintained until 3 days after surgery, which decreased 5 days after surgery. Five days after surgery, serum bilirubin values of the Control group and the Spleen group I were normal, and in the Portal vein group and the Spleen group II the values were higher than normal. In the linear comparison of each group and the pattern of the change of the entire group, significant differences were not shown (p=0.712) (Fig. 5).
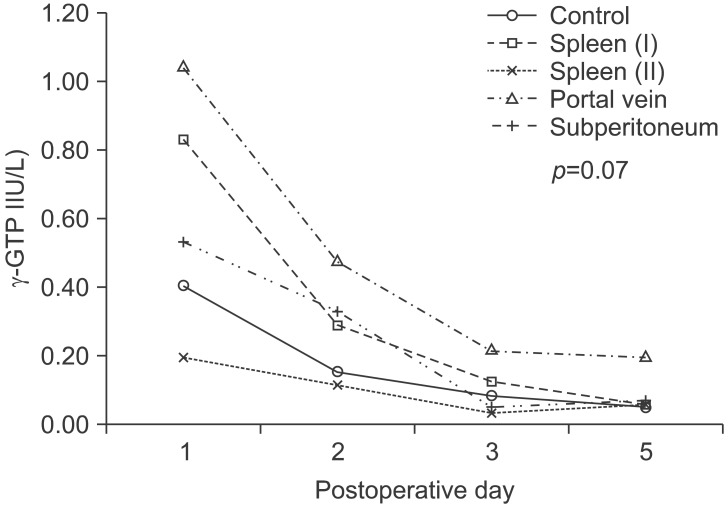
One day after surgery, blood γ-GTP was 0.19±0.10 IU/L, and only Spleen group II was lower than the 0.40±0.41 IU/L of the Control group. The Portal vein group was 2.03±2.52 IU/L which was highest, and the Spleen group I it was 0.83±1.36 IU/L, the Subperitoneal group was 0.53±0.31 IU/L, which was higher than the Control group, but statistical significance was not shown. Two and 3 days after surgery the blood γ-GTP pattern was almost identical to ALT; in the Portal vein group it was 0.47±0.45 IU/L and 0.21±0.16 IU/L, respectively, which were the highest. The Spleen group II was 0.11±0.09 IU/L and 0.03±0.02 IU/L, respectively, and the lowest values were shown. Five days after surgery, in contrast to other groups that showed the normal blood γ-GTP values of 0.05~0.07 IU/L, the Portal vein group was a high value of 0.19±0.16 IU/L. All groups showed a trend for decreasing values with time after surgery, but no statistical significance was detected (p=0.132). Comparison between each group demonstrated no statistical significance, while the Spleen group II and the Portal vein group showed a significant difference (p=0.059). The overall pattern of the change of the each group showed a pattern that was similar to ALT with time progression, however it was not statistically significant (p=0.070) (Fig. 6).
Five days after surgery, experimental rats were sacrificed and the liver tissues were extracted and examined macroscopically as well as microscopically. Macroscopically, hepatomegaly of the residual liver such as the omentum lobe and the right upper lobe could be observed, and liver cirrhosis was not observed. The result of the examination of each transplantation area showed that in the Spleen group II, the transplantation area of spleen showed edematous changes together with slight infarction findings. In the Subperitoneal group and the Portal vein group, morphological changes were not detected except for slight edema in the transplantation area.
In the histological findings of the Spleen group II and the Subperitoneal group, colonies of hepatocytes with abundant cytoplasm and small nuclei were detected in the spleen tissues and subperitoneal tissues that were extracted after sacrifice. In the liver tissues of the Portal vein group, surviving transplanted cells were present in the portal branches in addition to normal hepatocytes was observed, causing thrombi, and association with inflammation was observed (Fig. 7).
Acute liver failure refers to severe loss of the synthetic capacity of the liver, and hepatic coma caused by acute liver damages without previous liver disease. Clinically, acute liver failure is primarily caused by acute viral hepatitis or drug intoxication. In the surgical field, it is encountered in cases where failure of the recovery of liver function occurs after a massive hepatectomy.2 Once acute liver failure occurs, the mortality rate is higher than 80%, however, it has been reported in a prospective study that the survival rate of patients who received liver transplantation was 60~80%,9 and thus liver transplantation has been accepted as the only treatment procedure for acute liver failure.1 However, because of shortcomings such as insufficient donor livers, high postsurgical complications, and the side effects of immunosuppressants, studies on alternative treatments, symptomatic treatments, and treatment that may be a bridge until liver transplantation have been ongoing actively.345
As a procedure that creates acute liver failure experimentally, liver toxicity induction by injection of acetaminophen or carbon tetrachloride has been used primarily. This method has advantages in that it may be applied to experimental animals readily, however, it has shortcomings such as the fact that interval from liver injury to liver failure and death varies depending on age and individuals, and thus reproducibility is low.1011 Particularly, in this study of patients who received massive hepatectomy, liver dysfunction and liver failure were reproduced in an animal experimental model, and the usefulness of liver cell transplantation was assessed. Therefore, the different mechanism of liver injury using drugs, its high reproducibility can be pointed out as one of the main advantages. Total liver resection or partial liver resection methods have been used for liver resection. In the total hepatectomy cases, it appears that an environment identical to the graft failure of liver transplant in humans was provided, and thus the reproducibility may be highest. However, the fact that toxic materials or inflammatory factors were not secreted from the liver to the blood may be considered to be different from actual cases.11 Therefore, we conducted this experiment in an animal model in which a partial hepatectomy was performed.
In 1931, Higgins and Anderson8 reported an acute liver failure model that was obtained by ligating the lobe with sutures and subsequently performing 70% hepatectomy. This is a method that resects the middle lobe, left anterior lobe, right upper lobe, and right inferior lobe of liver, and leaves only the omental lobe. In the above cases, the shape of the right upper and inferior lobe covers the inferior vena cava, and thus if it is ligated with suture, the inferior vena cava may be injured and the rib may be extended excessively in order to provide a surgical space and thus good skills are required. In several later studies, hepatectomy in various ranges was used as liver failure models.12 One investigator reported that after 90% resection, the mortality rate was higher than 40% one week after surgery.1314 Recently, in another similar study, the average survival period after 90% resection was 3 days, and the survival rate 5 days after surgery was lower than 50%.15 Another recent series showed that in acute liver failure models, 95% liver resection have been reported.16 However, the mortality rate a short time after surgery was reported to be 80%, and thus this may not be a suitable model for investigating acute liver failure. In a most recent study, a laparotomy was performed prior to hepatectomy, the blood flow to the liver was partially blocked, released by re-laparotomy, and only 75% of the entire liver was resected.17 In our study, 90% hepatectomy was attempted to obtain acute liver failure models, however, the mortality rate after surgery increased noticeably, and thus 80% hepatectomy was performed which leaves the right upper lobe, and the inferior lobe was resected using surgical clips without affecting the inferior vena cava.
The method to isolate hepatocytes from experimental animals using collagenase was introduced for the first time in 1969 by Berry and Friend,18 and it became widespread as the basic surgical procedure in after being described in 1976 by Seglen.19 In many numerous later studies, experiments were conducted to isolate hepatocytes from rats, and which were transplanted to rats in which liver failure was induced by materials that are toxic to the liver, for example, dimethylnitrosamine, or liver dysfunction, which have been conducted for about 30 years. Even now, this method has been applied to evaluate the outcomes of liver cell transplantation or to assess prognosis. In our study, a modified Seglen's method was employed.
Liver cell transplantation in experimental animals was reported in 1977 for the first time.20 Although it has been applied clinically occasionally, the results have not been encouraging. Even now, numerous studies on the timing of hepatocyte transplantation, the injection volume, and the injection route are ongoing.21 In regard to liver cell transplantation after a partial hepatectomy, Gäbelein et al.15 reported that in cases in which 90% partial hepatectomy was performed, the group with transplanted liver cells 1 day prior to surgery showed a substantially higher survival rate than the group which received simultaneous liver cell transplantation and hepatectomy (72% vs. 29%). Clinically, it is difficult to perform liver cell transplantation prior to massive hepatectomy, and thus in our study 80% partial hepatectomy and liver cell transplantation were performed simultaneously. In several studies of liver cell transplantation, the number of hepatocytes to be transplanted was calculated and determined theoretically by the ratio of the liver weight to body weight. Generally, 5% of the liver weight that comprises the total body weight has been transplanted. For a rat that weighs 500 gm approximately 2×107 cells would be transplanted.1522 In our study, 2×106 or 2×107 viable cells were transplanted to determine the appropriate number of cells to be transplanted based on the Spleen group that has been used frequently in recent reports. When 2×107 cells were transplanted to the spleen, serum ALT was statistically different from the Control group in blood tests. Comparison of serum ALT and γ-GTP levels with the group injected with 2×106 cells showed no statistical significance, but a tendency for less elevated number of transplanted cells as observed. It is thought that in acute liver failure, transplanted cells play a role in facilitating recovery through regeneration of original hepatocytes.6 Such effects may be predicted in groups that show relatively low serum ALT values which implies liver injury after massive hepatectomy.
The number of transplant hepatocytes was calculated as 2×107 cells, and experiments according to varying routes of hepatocyte injection were performed. Reviewing the reports that examined the route of injection of hepatocytes, it has been reported that the environment is similar when the injection of hepatocytes is through the portal vein, and thus it is advantageous for grafting of transplanted cells.23 It is thought if a large number of cells are transplanted instead of injection through the portal vein, it would be significantly meaningful clinically to discover extrahepatic areas such as the spleen or the subperitoneal area that could be approached by a less invasive approach. In addition, it is considered that this method may avoid hepatic portal hypertension or thrombi formation that develops after injection of hepatocytes through the portal vein. Particularly, our results demonstrated elevation of serum ALT and γ-GTP values, as well as delayed recovery in the Portal vein group. It appears that the cause may be necrosis caused by hepatic portal hypertension or embolism. In addition, several reports described extrahepatic sites for liver cell transplantation such as the spleen, the peritoneal cavity, subcutaneous tissue layers, the pancreas, and the lung parenchyma. Except for the spleen and the peritoneal cavity, shortcomings are present that in most sites, in which the engraft of transplanted cells is unstable, and the quantity of transplanted cells is not constant. Even after engrafting, it has been pointed out that the cell survival period is short. When cells are transplanted to the spleen, the transplanted cells migrate to the liver through the splenic medulla and block the portal vein branch, and which may exert adverse effects on the already damaged liver.6121421 In our study, liver cells were transplanted through the portal vein, the spleen, and the subperitoneum, and in the Spleen group, positive results were obtained. Moreover, in our study the liver was transplanted to a liver failure rat model, and to evaluate the results, indirect methods through blood tests were used. In the future, it appears that to obtain more direct results, transplant cells labeling with isotopes, or using gene knockout animals would be recommended.
Although the number of study subjects was small, a liver failure model was created, and the effectiveness of the number of cells and the injection sites was evaluated using an experimental animal model. In the group to which 2×107 liver cells were transplanted to the spleen, good results were obtained in comparison with the Control group. It appears that studies on a larger number of cases are required, and for its clinical application, studies on the storage of hepatocytes after isolation and the route for the actual application are required. Particularly, in the current experiment animal model, it was difficult to compare survival rates. It is thus is thought that more studies on experimental animal models should be performed.
References
1. Gill RQ, Sterling RK. Acute liver failure. J Clin Gastroenterol. 2001; 33:191–198. PMID: 11500606.

2. Gotthardt D, Riediger C, Weiss KH, et al. Fulminant hepatic failure: etiology and indications for liver transplantation. Nephrol Dial Transplant. 2007; 22(Suppl 8):viii5–viii8. PMID: 17890263.

3. Debray D, Yousef N, Durand P. New management options for end-stage chronic liver disease and acute liver failure: potential for pediatric patients. Paediatr Drugs. 2006; 8:1–13. PMID: 16494508.
4. Kjaergard LL, Liu J, Als-Nielsen B, Gluud C. Artificial and bioartificial support systems for acute and acute-on-chronic liver failure: a systematic review. JAMA. 2003; 289:217–222. PMID: 12517233.
5. Demetriou AA, Brown RS Jr, Busuttil RW, et al. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann Surg. 2004; 239:660–667. PMID: 15082970.

6. Bumgardner GL, Fasola C, Sutherland DE. Prospects for hepatocyte transplantation. Hepatology. 1988; 8:1158–1161. PMID: 3047038.

7. Strom SC, Fisher RA, Thompson MT, et al. Hepatocyte transplantation as a bridge to orthotopic liver transplantation in terminal liver failure. Transplantation. 1997; 63:559–569. PMID: 9047152.

8. Higgins GM, Anderson RM. Experimental pathology of the liver I Restoration of the liver of the white rat following surgical removal. Arch Pathol. 1931; 12:186–202.
9. Ostapowicz G, Fontana RJ, Schiødt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002; 137:947–954. PMID: 12484709.

10. Nardo B, Caraceni P, Puviani L, et al. Successful treatment of CCl4-induced acute liver failure with portal vein arterialization in the rat. J Surg Res. 2006; 135:394–401. PMID: 16780880.

11. Tuñón MJ, Alvarez M, Culebras JM, González-Gallego J. An overview of animal models for investigating the pathogenesis and therapeutic strategies in acute hepatic failure. World J Gastroenterol. 2009; 15:3086–3098. PMID: 19575487.

12. Fuller BJ. Transplantation of isolated hepatocytes. A review of current ideas. J Hepatol. 1988; 7:368–376. PMID: 3069902.
13. Demetriou AA, Reisner A, Sanchez J, Levenson SM, Moscioni AD, Chowdhury JR. Transplantation of microcarrier-attached hepatocytes into 90% partially hepatectomized rats. Hepatology. 1988; 8:1006–1009. PMID: 3047034.

14. Demetriou AA, Felcher A, Moscioni AD. Hepatocyte transplantation. A potential treatment for liver disease. Dig Dis Sci. 1991; 36:1320–1326. PMID: 1893819.
15. Gäbelein G, Nüssler AK, Morgott F, et al. Intrasplenic or subperitoneal hepatocyte transplantation to increase survival after surgically induced hepatic failure? Eur Surg Res. 2008; 41:253–259. PMID: 18577870.

16. He Y, Zhou J, Dou KF, Chen Y. A rat model for acute hepatic failure. Hepatobiliary Pancreat Dis Int. 2003; 2:423–425. PMID: 14599952.
17. Roger V, Balladur P, Honiger J, et al. A good model of acute hepatic failure: 95% hepatectomy. Treatment by transplantation of hepatocytes. Chirurgie. 1996; 121:470–473. PMID: 8978143.
18. Berry MN, Friend DS. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969; 43:506–520. PMID: 4900611.
19. Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976; 13:29–83. PMID: 177845.
20. Groth CG, Arborgh B, Björkén C, Sundberg B, Lundgren G. Correction of hyperbilirubinemia in the glucuronyltransferase-deficient rat by intraportal hepatocyte transplantation. Transplant Proc. 1977; 9:313–316. PMID: 405772.
21. Hillan KJ, Burt AD, George WD, MacSween RN, Griffiths MR, Bradley JA. Intrasplenic hepatocyte transplantation in rats with experimental liver injury: morphological and morphometric studies. J Pathol. 1989; 159:67–73. PMID: 2809886.

22. Liu XL, Li LJ, Chen Z. Isolation and primary culture of rat hepatocytes. Hepatobiliary Pancreat Dis Int. 2002; 1:77–79. PMID: 14607628.
23. Gupta S, Rajvanshi P, Lee CD. Integration of transplanted hepatocytes into host liver plates demonstrated with dipeptidyl peptidase IV-deficient rats. Proc Natl Acad Sci U S A. 1995; 92:5860–5864. PMID: 7597042.

Fig. 1
Operative findings after massive resection of liver (arrow head: right superior lobe and omental lobe).
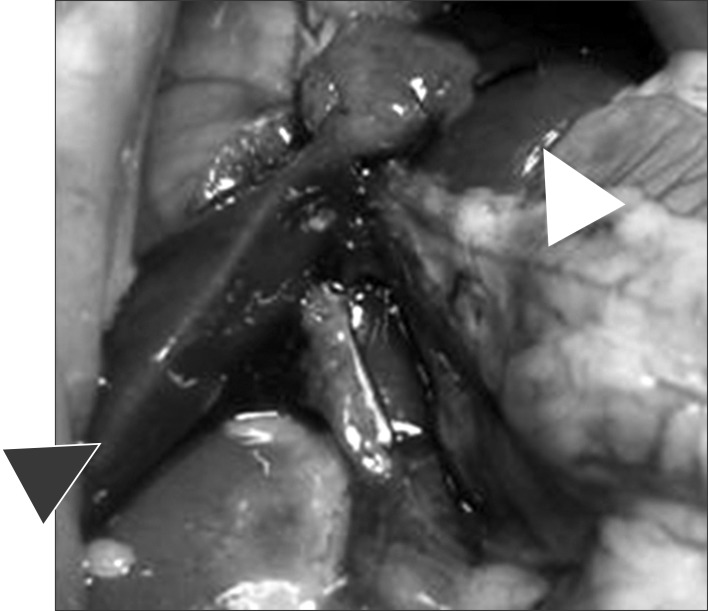
Fig. 2
Operative view of canulation in portal vein (A), infusion of collagenase solution with whitish discoloration of liver (B) and resected liver after collagenase perfusion (C).

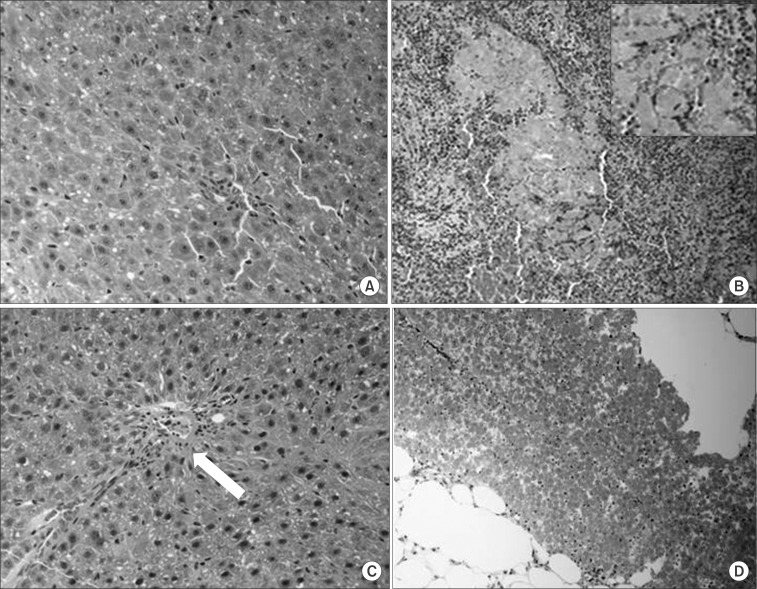
Fig. 7
Histologic feature of hepatocyte of each groups on the day of sacrifice. (A) Liver of control group (HE staining, ×400), (B) colonization of the irregular sheets of transplanted hepatocyte in spleen group II (some of which show degenerative feature) (HE staining ×200 and ×400), (C) transplanted hepatocyte in portal vein and occlusion of smaller portal branch on portal vein group, (HE staining ×400), (D) colonization of transplanted hepatocyte between subperitoneal fat tissues on subperitoneal group (HE staining, ×200), (arrow: hepatocytes & red cells in portal vein branch).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download