Abstract
Reconstructing the impact of infectious disease on past populations is one of the main fields in paleopathological studies. The initial phase of paleopathology was descriptive, focusing on the identification and presence of disease in the past. However, currently paleopathological studies are moving toward probing questions about the larger picture of origin and transmission of disease agents. In this study, paleopathological studies of major infectious disease (i.e., tubuerculosis, treponemal disease and leprosy) were reviewed through osteoarcheological work published in American Journal of Physical Anthropology, International Journal of Osteoarchaeology, Journal of Archaeological Science and International Journal of Paleopathology from 1981 to 2017. A basic objective of this research was to examine many types of research in paleopathology and to characterize research trend in this field. As paleopathological studies becomes more abundant, the approaches to infectious disease have been increasingly specialized and interdisciplinary from 1980. Also, methodology used in paleopathology continues to evolve through the holistic approaches of molecular analysis, radiology and histopathology. Ultimately, this study reinforces the importance for retention of large-scale skeletal collections for paleopathological study in population perspective. In the near future, Korean paleopathology can contribute in the reconstructions of the history of disease and its effect on past human populations.
Go to : 
References
1. Inhorn MC, Brown PJ. The anthropology of infectious disease. Annu Rev Anthropol. 1990; 19:89–117.

2. Ortner DJ. Differential diagnosis of skeletal lesions in infectious disease. Pinhasi R, Mays S, editors. editors.Advances in human palaeopathology. Chichester, England: John Wiley & Sons, Ltd;2008. p. 189–214.

3. Molnar E, Palfi G. Probable cases of skeletal infections in the 17th century anthropological series from Bácsalmás (Hungary). Acta Biol Szeged. 1994; 40:117–33.
4. Armelagos GJ, Brown PJ, Turner B. Evolutionary, historical and political economic perspectives on health and disease. Soc Sci Med. 2005; 61:755–65.

5. Harper KN, Zuckerman MK, Harper ML, Kingston JD, Armelagos GJ. The origin and antiquity of syphilis revisited: an appraisal of Old World pre-Columbian evidence for treponemal infection. Am J Phys Anthropol. 2011; 146(Suppl 53):S99–133.

6. Zuckerman MK, Harper KN. Paleoepidemiological and biocultural approaches to ancient disease: the origin and antiquity of syphilis. Zuckerman MK, Martin DL, editors. editors.New directions in biocultural anthropology. New Jersey: John Wiley & Sons;2016. p. 317–35.

7. Monot M, Honore N, Garnier T, Araoz R, Coppee JY, Lacroix C, et al. On the origin of leprosy. Science. 2005; 308:1040–2.

8. Stone AC, Wilbur AK, Buikstra JE, Roberts CA. Tuberculosis and leprosy in perspective. Am J Phys Anthropol. 2009; 140(Suppl 49):S66–94.

9. Suzuki T, Fujita H, Choi JG. New evidence of tuberculosis from prehistoric Korea-Population movement and early evidence of tuberculosis in Far East Asia. Am J Phys Anthropol. 2008; 136:357–60.
10. Mays S. A perspective on human osteoarchaeology in Brit-ain. Int J Osteoarchaeol. 1997; 7:600–4.

11. Mays S. Human osteoarchaeology in the UK 2001–2007: a bibliometric perspective. Int J Osteoarchaeol. 2000; 20:192–204.

12. Ortner DJ, Putschar W. Identification of pathological conditions in human skeletal remains. Washington: Smithsonian Institution Press;1985. p. 130.
13. Spigelman M, Lemma E. The use of the polymerase chain reaction (PCR) to detect mycobacterium tuberculosis in ancient skeletons. Int J Osteoarchaeol. 1993; 3:137–43.
14. Braun M, Pfeiffer S. DNA from mycobacterium tuberculosis complex identified in North American, Pre-Columbian human skeletal remains. J Archaeol Sci. 1998; 25:271–7.
15. Comas I, Coscolla M, Luo T, Borrell S, Holt KE, Kato-Maeda M, et al. Out-of-Africa migration and Neolithic coexpansion of mycobacterium tuberculosis with modern humans. Nat Genet. 2013; 45:1176–82.

16. Ortner DJ. Paleopathology: implications for the history and evolution of tuberculosis. Pálfi G, Dutour O, Deák J, Hutás IS, editors. editors.Tuberculosis: past and present. Hungary: Golden Book Publisher Ltd;1999. p. 255–61.
17. Morse D. Pre-historic tuberculosis in America. Am Rev Respir Dis. 1961; 83:489–504.
18. Morse D. On tuberculosis. Brothwell D, Sandison AT, editors. editors.Diseases in antiquity. Springfield: Charles C. Tho-mas;1967. p. 249–71.
19. Ritchie WA. Paleopathological evidence suggesting preColumbian tuberculosis in New York State. Am J Phys Anthropol. 1952; 10:305–11.

20. Lichtor J, Lichtor A. Paleopathological evidence suggesting pre-Columbian tuberculosis of the spine. J Bone Joint Surg. 1957; 39:1398–9.

21. Holloway KL, Henneberg R, de Barros Lopes M, Henneberg M. Evolution of human tuberculosis: a systematic review and metaanalysis of paleopathological evidence. HOMO-J Comp Hum Biol. 2011; 62:402–58.

22. Salo WL, Aufderheide AC, Buikstra J, Holcomb TA. Iden-tification of mycobacterium tuberculosis DNA in a preColumbian Peruvian mummy. Proceedings of the National Academy of Sciences. 1994; 91:2091–4.

23. Arriaza BT, Salo WL, Aufderheide AC, Holcomb TA. Pre-Columbian tuberculosis in Northern Chile: molecular and skeletal evidence. Am J Phys Anthropol. 1995; 98:37–45.

24. Bos KI, Harkins KM, Herbig A, Coscolla M, Weber N, Comas I, et al. Pre-Columbian mycobacterial genomes reveal seals as a source of New World human tuberculosis. Nature. 2014; 514:494–7.

25. Suzuki T. Paleopathological study on infectious disease in Japan. Ortner DJ, Aufderheide AC, editors. editors.Human paleo-pathology: current syntheses and future options. Washington DC: Smithsonian Institution Press;1991. p. 128–39.
26. Pechenkina EA, Benfer RA Jr, Ma X. Diet and health in the Neolithic of the Wei and Middle Yellow River Basins, Northern China. In: Cohen MN, Crane-Kramer GNM, edi-tors. Ancient health: skeletal indicators of agricultural and economic intensification. Gainesville: University of Florida Press;2007. p. 255–72.
27. Suzuki T, Inoue T. Earliest evidence of spinal tuberculosis from the aneolithic Yayoi period in Japan. Int J Osteoar-chaeol. 2007; 17:392–402.

28. Blau S, Yagodin V. Osteoarchaeological evidence for leprosy from Western Central Asia. Am J Phys Anthropol. 2005; 126:150–8.

29. Tayles N, Buckley HR. Leprosy and tuberculosis in Iron Age southeast Asia? Am J Phys Anthropol. 2004; 125:239–56.

30. Robbins G, Tripathy VM, Misra VN, Mohanty RK, Shinde VS, Gray KM, et al. Ancient skeletal evidence for leprosy in India (2000 BC). PLoS One. 2009; 4:e5669.

31. Taylor GM, Blau S, Mays S, Monot M, Lee OYC, Minnikin DE, et al. Mycobacterium leprae genotype amplified from an archaeological case of lepromatous leprosy in Central Asia. J Archaeol Sci. 2009; 36:2408–14.

32. Suzuki K, Takigawa W, Tanigawa K, Nakamura K, Ishido Y, Kawashima A, et al. Detection of Mycobacterium leprae DNA from archaeological skeletal remains in Japan using whole genome amplification and polymerase chain reaction. PLoS One. 2010; 5:e12422.

33. Barnes I, Thomas MG. Evaluating bacterial pathogen DNA preservation in museum osteological collections. Proceedings of the Royal Society of London B: Biological Sciences. 2006; 273:645–53.

34. Bouwman AS, Brown TA. The limits of biomolecular pal-aeopathology: ancient DNA cannot be used to study vene-real syphilis. J Archaeol Sci. 2005; 32:703–13.

35. Kolman CJ, Centurion-Lara A, Lukehart SA, Owsley DW, Tuross N. Identification of Treponema pallidum subspecies pallidum in a 200-year-old skeletal specimen. J Infect Dis. 1999; 180:2060–3.
36. Mark L, Gulyas-Fekete G, Marcsik A, Molnar E, Palfi G. Analysis of ancient mycolic acids by using MALDI TOF MS: response to “Essentials in the use of mycolic acid biomarkers for tuberculosis detection” by Minnikin et al., 2010. J Archaeol Sci. 2011; 38:1111–8.
37. Roberts CA, Millard AR, Nowell GM, Gröcke DR, Macpherson CG, Pearson DG, et al. Isotopic tracing of the impact of mobility on infectious disease: the origin of people with treponematosis buried in Hull, England, in the late medieval period. Am J Phys Anthropol. 2013; 150:273–85.

Go to : 
Table 1.
Journals included in this study
Table 2.
Classification of articles examined in this study
Table 3.
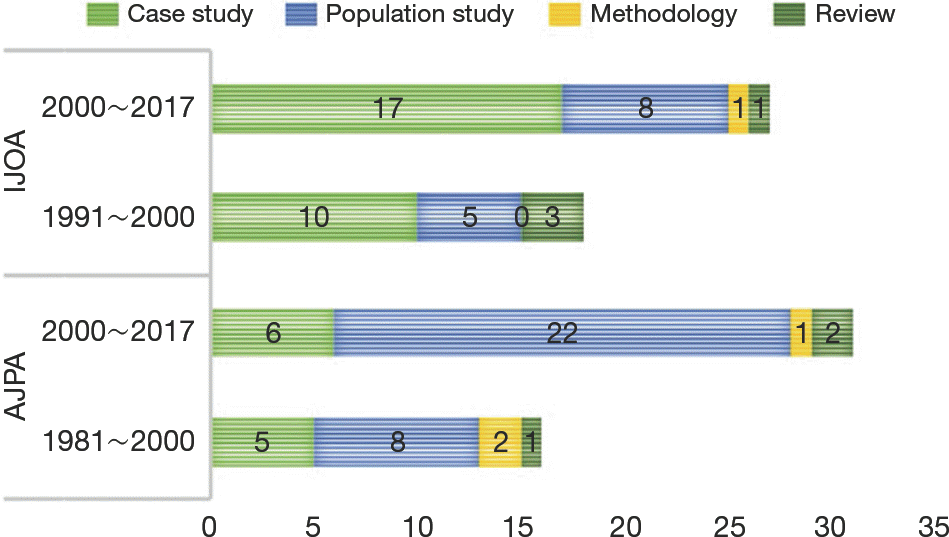
Articles from major anthropological journals split by ar-ticle categories
Table 4.
Methodology used in the articles by journal and period
Table 5.
Methodology of the articles split by infectious disease




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download