Abstract
Critical limb ischemia (CLI) is the most severe peripheral artery disease and caused by thrombus formation in blood vessel. The current strategies for treating CLI does not protect limb amputation and reduction in the risk of mortality. Recently, human bone marrow derived mesenchymal stem cells (BD-MSC) were reported to have a paracrine effects on angiogenesis in several ischemic diseases. So, we validate to determine whether BD-MSC protect against ferric chloride treated CLI and induce angiogenesis.
To characterized human bone marrow derived stem cell, BD-MSC differentiated to osteocytes and adipocytes and validated stemness using flow cytometry. Endothelial cell induced angiogenesis followed by mesenchymal stem cell cultured medium treatment in HUVEC in vitro. We also mimicked CLI patients condition using FeCl3 treated CLI mouse and injected one hundred thousand of BD-MSC along the femoral artery to leg muscle. We validated stem cell survival, blood vessel formation, leg muscle condition and fibrosis compared by saline injected mice 28 days later.
In this study, BD-MSC cultured medium treatment increased migration and tube formation of HUVEC and BD-MSC injection had an effective blood vessel formation in FeCl3 treated CLI. As well as blood vessel formation, limb salvage rate also improved and fibrosis area statistically decreased in BD-MSC injected mice.
In conclusion, bone marrow derived mesenchymal stem cell improved not only blood vessel formation but also reduction of fibrosis in FeCl3 treated CLI mice and finally protected limb amputation.
Go to : 
References
1. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007; 45(Suppl S):S5–67.

2. Weitz JI, Byrne J, Clagett GP, Farkouh ME, Porter JM, Sackett DL, et al. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: a critical review. Circulation. 1996; 94:3026–49.

4. Burggraf D, Vosko MR, Schubert M, Stassen JM, Hamann GF. Different therapy options protecting microvasculature after experimental cerebral ischaemia and reperfusion. Thromb Haemost. 2010; 103:891–900.

6. Söder HK, Manninen HI, Jaakkola P, Matsi PJ, Räsänen HT, Kaukanen E, et al. Prospective trial of infrapopliteal artery balloon angioplasty for critical limb ischemia: angiographic and clinical results. J Vasc Interv Radiol. 2000; 11:1021–31.

7. Nasr MK, McCarthy RJ, Hardman J, Chalmers A, Horrocks M. The increasing role of percutaneous transluminal angioplasty in the primary management of critical limb ischaemia. Eur J Vasc Endovasc Surg. 2002; 23:398–403.

8. Silvestre JS, Lévy BI. Angiogenesis therapy in ischemic disease. Arch Mal Coeur Vaiss. 2002; 95:189–96.
9. Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, et al. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989; 321:1174–78.

10. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002; 13:4279–95.

11. Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008; 103:1204–19.

12. Hocking AM, Gibran NS. Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res. 2010; 316:2213–19.

13. Chen L, Tredget EE, Wu PYG, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE. 2008; 3:e1886.

14. Limbourg A, Korff T, Napp LC, Schaper W, Drexler H, Limbourg FP, et al. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hindlimb ischemia. Nat Protoc. 2009; 4:1737–46.

15. Kurz KD, Main BW, Sandusky GE. Rat model of arterial thrombosis induced by ferric chloride. Thromb Res. 1990; 60:269–80.

16. Ni H, Denis CV, Subbarao S, Degen JL, Sato TN, Hynes RO, et al. Persistence of platelet thrombus formation in arterioles of mice lacking both von willebrand factor and fibrinogen. J Clin Invest. 2000; 106:385–92.

17. Surin WR, Prakash P, Barthwal MK, Dikshit M. Optimization of ferric chloride induced thrombosis model in rats: effect of antiplatelet and anticoagulant drugs. J Pharmacol Toxicol Methods. 2010; 61:287–91.

18. Mareschi K, Biasin E, Piacibello W, Aglietta M, Madon E, Fagioli F, et al. Isolation of human mesenchymal stem cells: bone marrow versus umbilical cord blood. Haematologica. 2001; 86:1099–100.
19. Jaiswal RK, Jaiswal N, Bruder SP, Mbalaviele G, Marshak DR, Pittenger MF, et al. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J Biol Chem. 2000; 275:9645–52.

20. Cai L, Johnstone BH, Cook TG, Tan J, Fishbein MC, Chen PS, et al. IFATS collection: Human adipose tissue-derived stem cells induce angiogenesis and nerve sprouting following myocardial infarction, in conjunction with potent preservation of cardiac function. Stem Cells. 2009; 27:230–7.

21. van Velthoven CT, Kavelaars A, Heijnen CJ. Mesenchymal stem cells as a treatment for neonatal ischemic brain damage. Pediatr Res. 2012; 71:474–81.

22. Ramot Y, Meiron M, Toren A, Steiner M, Nyska A. Safety and bio distribution profile of placental-derived mesenchymal stromal cells (PLX-PAD) following intramuscular delivery. Toxicol Pathol. 2009; 37:606–16.
Go to : 
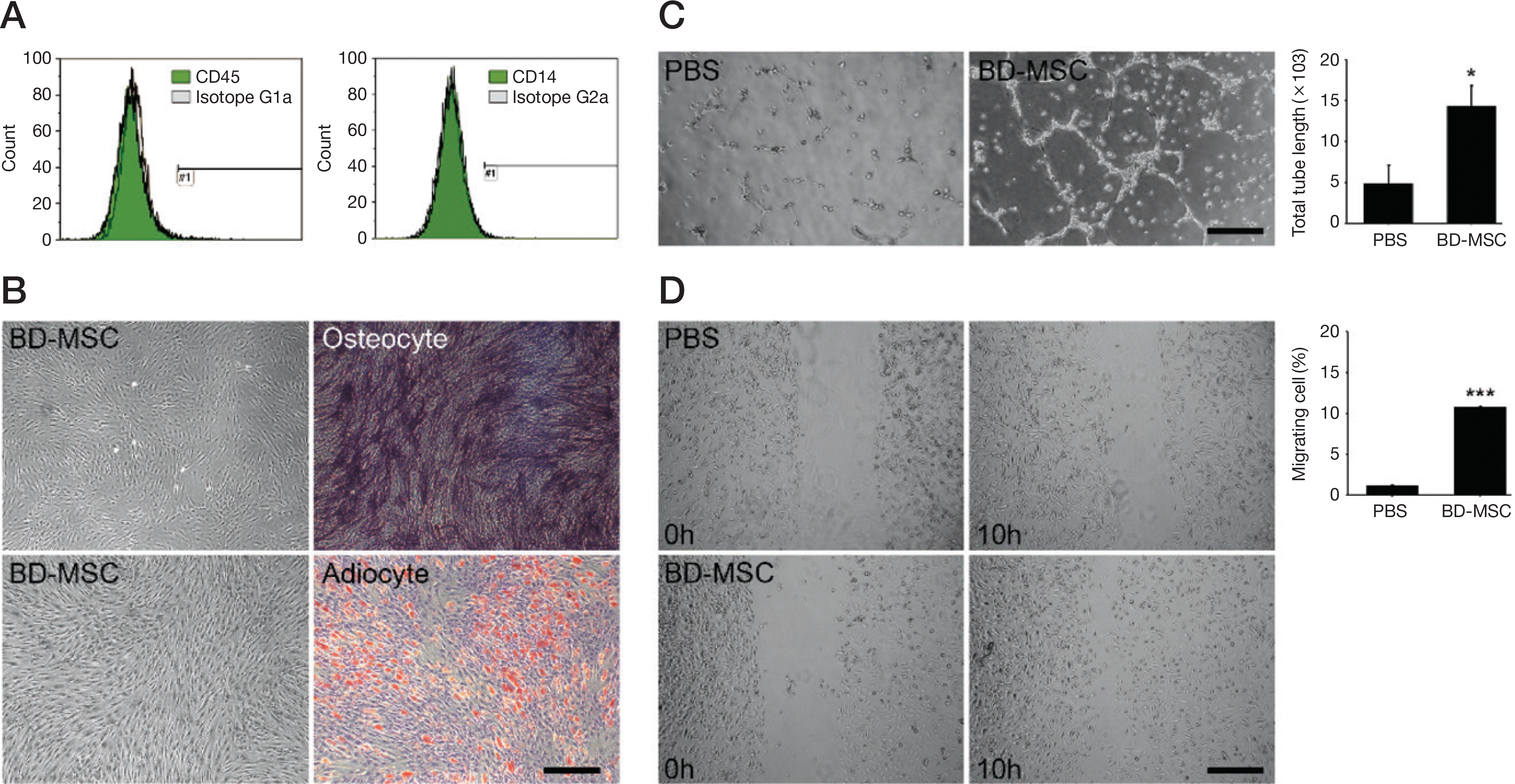 | Fig. 1.Characterization of Bone marrow derived mesenchymal stem cell and validation of angiogenesis in vitro. A. Flow cytometry analysis showed BD-MSC of negative stem cell markers such as CD45 and CD14 expression. B. BD-MSC of left panel differentiated to os-teocytes (right, top) and adipocytes (right, bottom) respectively and the differentiated cells stained with alkaline phosphatase and Oil red O respectively. Magnification = × 40. C. HUVEC showed tube formation in growth factor reduced Matrigel assay after PBS treated (PBS) or BD-MSC incubated medium (BD-MSC). Magnification = × 40. D. HUVEC showed wound migration assay after PBS treated (PBS) or BD-MSC incubated medium (BD-MSC). Scale bar = 1,000 pixel, ∗p<0.05, ∗∗p<0.01 vs. PBS treated HUVEC. PBS: phosphate buffer saline, BD-MSC: bone marrow-derived mesenchymal stem cell. |
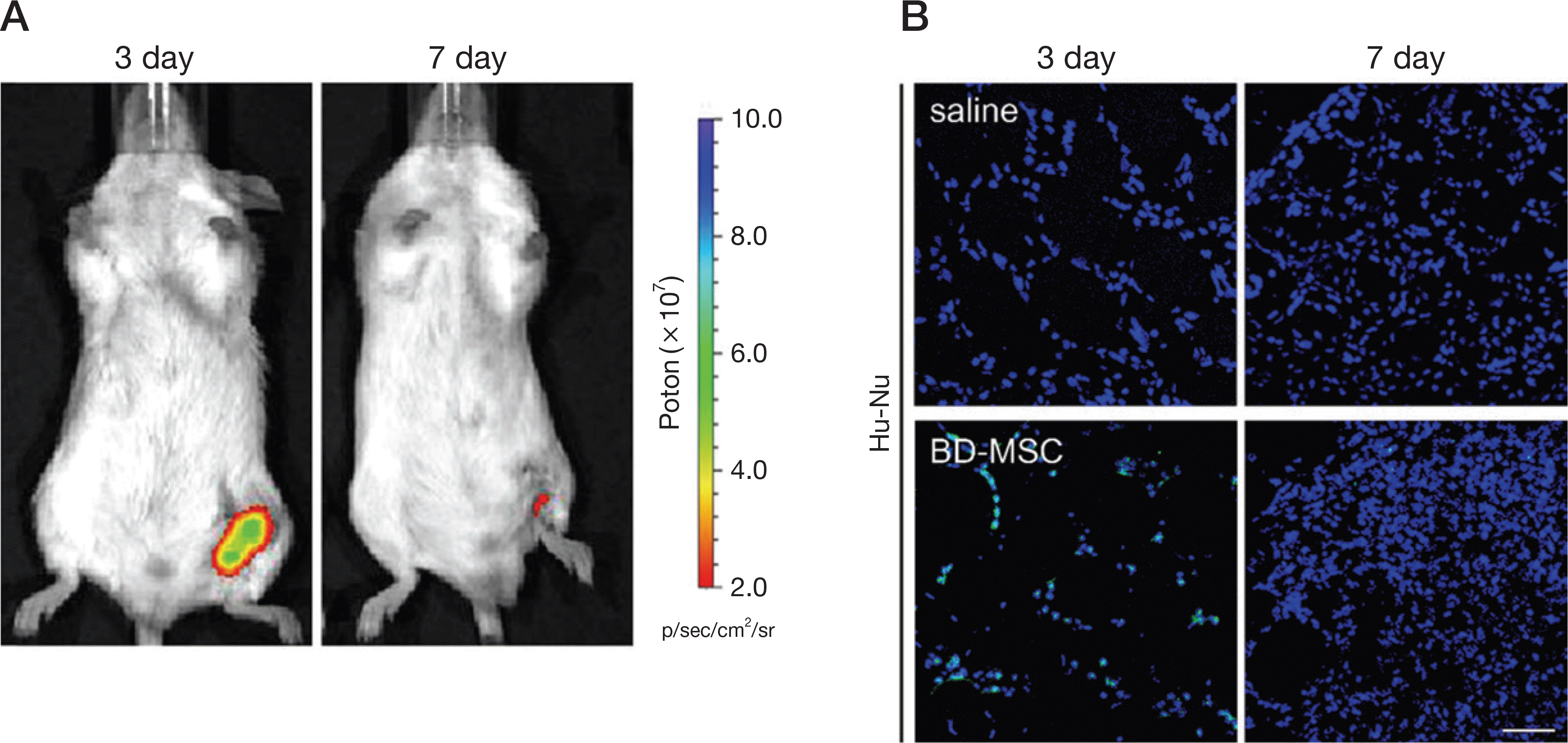 | Fig. 2.Validation of injected BD-MSC survival to FeCl3 treated mouse model. A. In vivo images showed expression of injected BD-MSC in FeCl3 treated mouse leg (BD-MSC) after 3 and 7 days. B. Confocal microscopy images showed Hu-Nu intensity of saline injected (saline) or BD-MSC injected (BD-MSC) in FeCl3 treated mouse model after 3 and 7 days (green: Hu-Nu, blue: DAPI (nucleus)). Scale bar = 50 μm, Hu-Nu: human nuclei, BD-MSC: bone marrow-drived mesenchymal stem cell. |
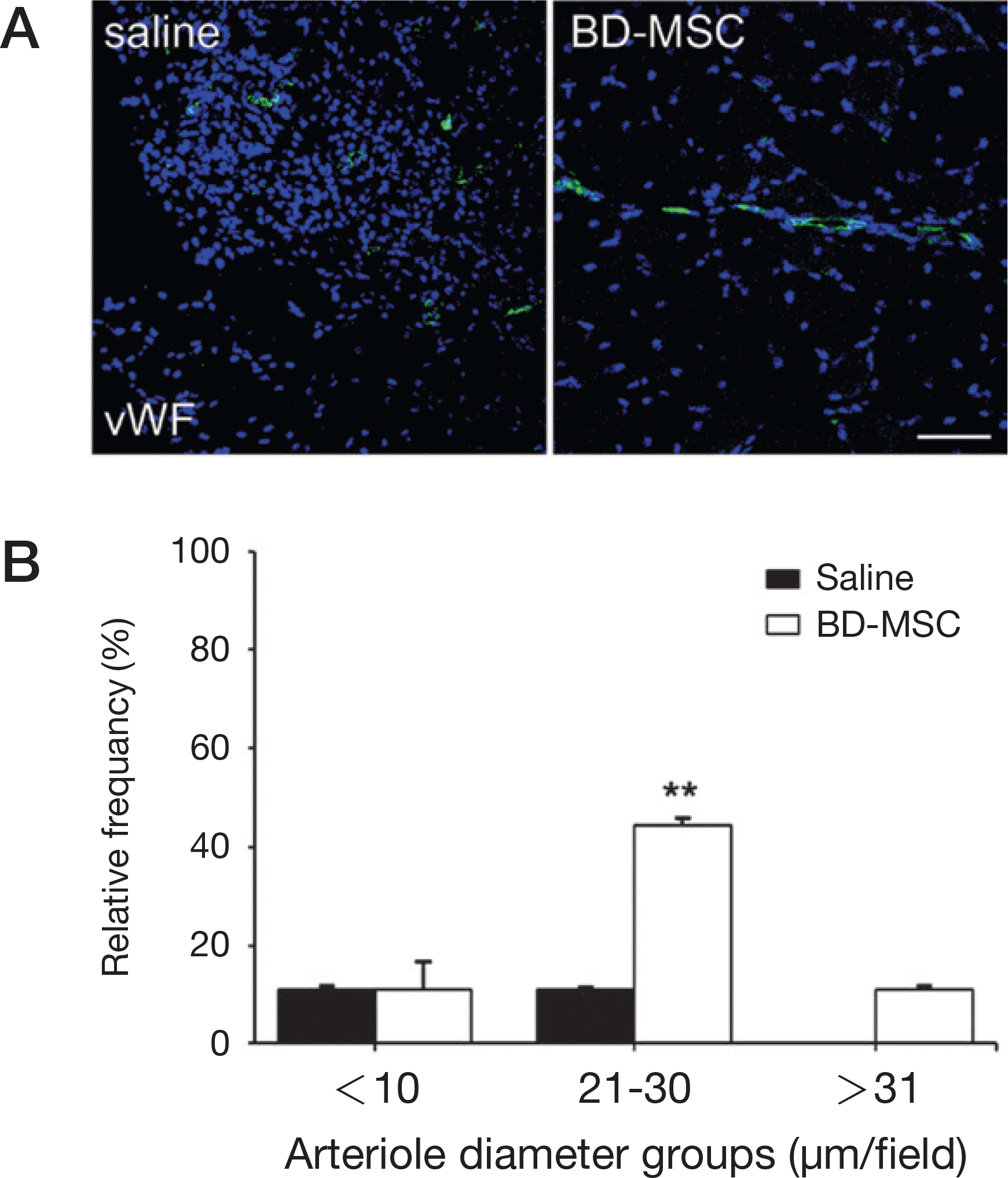 | Fig. 3.Validation of angiogenesis in FeCl3 treated mouse model. A. Confocal microscopy images showed vWF (endothelial cells) intensity of saline injected (saline) or BD-MSC injected (BD-MSC) in FeCl3 treated mouse model (green: vWF, blue: DAPI (nucleus)). Scale bar = 50 μm. B. Density for each group of blood vessels: under 10, 10–20, 20–30 and above 31 μm/field was obtained from vWF stained slides and measured by Zen software. ∗∗P<0.01 vs. saline treated mice. BD-MSC: bone marrow-derived mesenchymal stem cell, vWF: von Willebrand factor. |
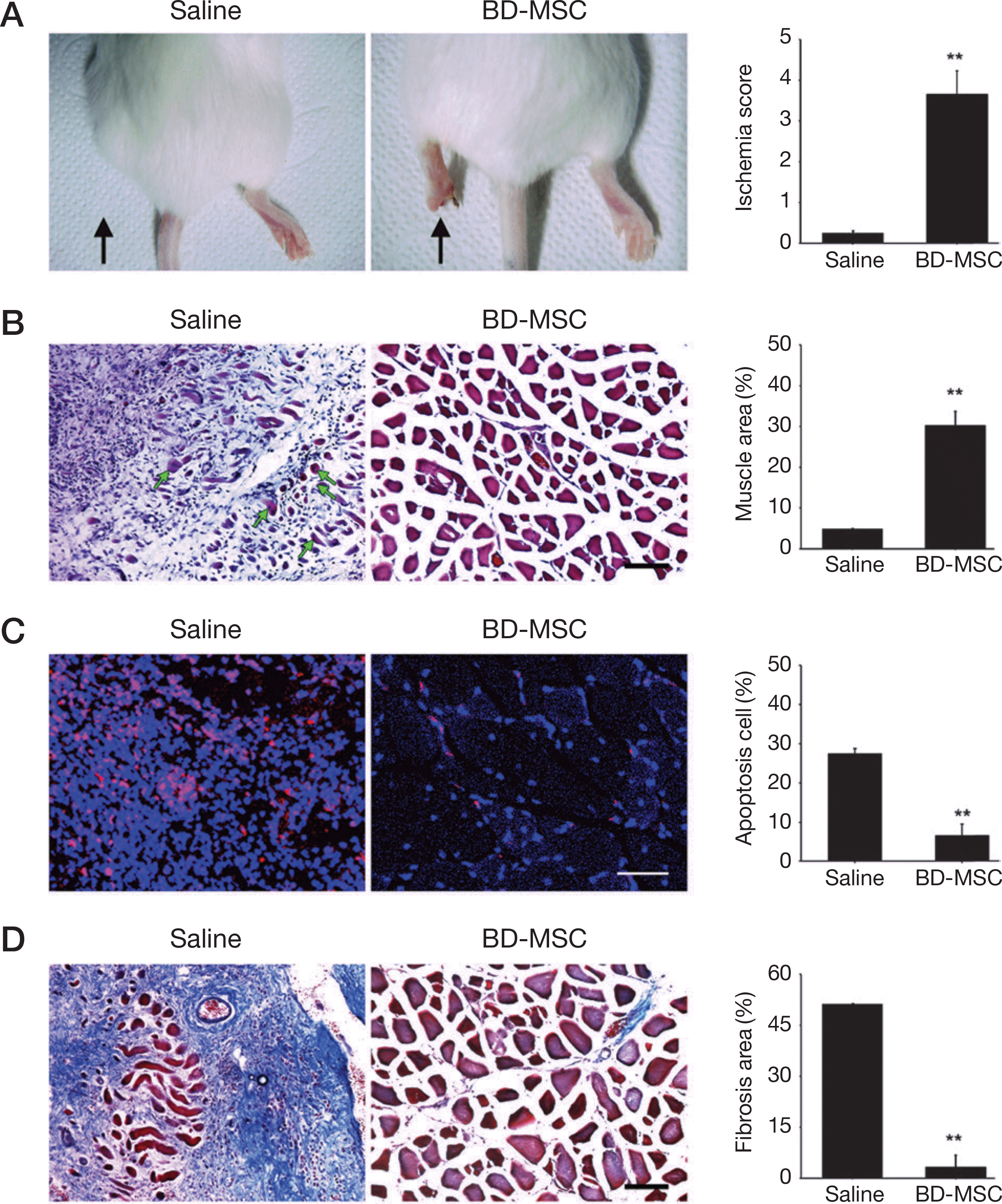 | Fig. 4.Validation of BD-MSC protection from FeCl3 induced damage. A. left side of leg appearance showed skin color and limb condition of saline injected (saline) or BD-MSC injected (BD-MSC) in FeCl3 treated mouse model (arrow) and graph indicated improved functional outcomes in BD-MSC injected group. B. Central nucleus of damaged skeletal muscles was indicated (green arrow) and confirmed by Hematoxylin and Eosin staining. C. Confocal fluorescence analysis showed apoptosis cell (red) in FeCl3 treated mouse model. D. Fibrous (blue) was validated by Masson's trichrome in FeCl3 induced muscle. Scale bar=100 μm, ∗P<0.01 vs. saline treated mice. |
Table 1.
Ischemia scale used for functional test of limb ischemia leg




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download