Abstract
Thrmobospondin-1 is the multifunctional protein that modulates endothelial cell and tumor cell behavior via several cell surface receptors and inhibits angiogenesis. In vitro, thrombospondin-1 alters adhesion, proliferation, motility, and survival of endothelial and cancer cells. Studies have confirmed that increased TSP-1 expression suppresses growth or metastasis of some tumors and inhibits angiogenesis. In the past three decades, inhibitors of angiogenesis have been developed as regulators target the vascular endothelial growth factor (VEGF) signaling pathway and small molecule tyrosine kinase inhibitors have been clinically approved. TSP-1 has several functional domain structures and inhibits tumor angiogenesis by engaging receptors CD36 and CD47. TSP-1 binding to CD47 dissociates it from VEGFR2, inhibiting downstream AKT activation and functional responses of endothelial cells to VEGF. Recently, macrophage phagocytosis and cytotoxic T-cell induction of tumor cells mediated by CD47-specific blocking antibodies have been proposed. These findings provide a new therapeutic paradigm for elinination of cancer cells and inhibition of angiogenesis of tumor by TSP-1.
Go to : 
References
1. Folkman J. Tumor angiogenesis: therapeutic implications. New Eng J Med. 1971; 285:1182–6.
2. Cook KM, Figg WD. Angiogenesis inhibitors: current strategies and future prospects. CA Cancer J Clin. 2010; 60:222–43.

4. Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990; 82:4–6.

5. Verheul HM, Voest EE, Schlingemann RO. Are tumors an-giogenesis-dependent? J Pathol. 2004; 202:5–13.
6. Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007; 6:273–86.

7. Faivre S, Demetri G, Sargent W, Raymond E. Molecular basis for sunitinib efficacy and future clinical development. Nat Rev Drug Discov. 2007; 6:734–45.

8. Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006; 5:835–44.

9. Motzer RJ, Bukowski RM. Targeted therapy for metastatic renal cell carcinoma. J Clin Oncol. 2006; 24:5601–8.

10. Bergers G, Hanahan D. Modes of resistance to antiangiogenic therapy. Nat Rev Cancer. 2008; 8:592–603.

11. Verheul HM, Pinedo HM. Possible molecular mechanisms involved in the toxicity of angiogenesis inhibition. Nat Rev Cancer. 2007; 7:475–85.

12. Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res. 2008; 79:581–8.

13. Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008; 15:261–71.

14. Wang S, Olson EN. AngiomiRs-key regulators of angiogenesis. Curr Opin Genet Dev. 2009; 19:205–11.

15. Albelda SM, Role of integrins and other cell adhesion molecules in tumor progression and metastasis. Lab Invest. 1993; 68:4–17.
16. Roberts DD. Regulaton of tumor growth and metastasis by thrombospondin-1. FASEB J. 1996; 10:1183–91.
17. Morandi V, Fauvel-Lafeve F, Legrand C, Legrand YJ. Role of thrombospondin in the adhesion of human endothelial cells in primary culture. In Vitro Cell Dev Biol Anim. 1993; 29:585–91.

18. Dreyfus M, Lahav J. The buildup of the thrombospondin extracellular matrix. An apparent dependence on synthesis and on preformed fibrillar matrix. Eur J Cell Biol. 1988; 47:275–82.
19. Lawler J. The functions of thrombospondin-1 and −2. Curr Opin Cell Biol. 2000; 12:634–40.
20. Wang TN, Qian XH, Granick MS, Solomon MP, Rothman VL, Tuszynski GP. The effect of thrombospondin on oral squamous carcinoma cell invasion of collagen. Am J Surg. 1995; 170:502–5.

21. Lawler J, Connolly JE, Ferro P, Derick LH. Thrombin and chymotrypsin interactions with thrombospondin. Ann NY Acd Sci. 1986; 485:273–87.

22. Bein K, Simons M. Thrombospondin type 1 repeats interact with matrix metalloproteinase 2. Regulation of metalloproteinase activity. J Biol Chem. 2000; 275:32167–73.
23. Iruela-Arispe ML, Lombardo M, Krutzsch HC, Lawler J, Roberts DD. Inhibition of angiogenesis by thrombospondin1 is mediated by 2 independent regions within the type 1 repeats. Circulation. 1999; 100:1423–31.

24. Chen H, Strickland DK, Mosher DF. Metabolism of thrombospondin 2. Binding and degradation by 3t3 cells and glycosaminoglycan-variant Chinese hamster ovary cells. J Biol Chem. 1996; 271:15993–9.
25. Murphy-Ullrich JE, Schultz-Cherry S, Höök M. Transforming growth factor-beta complexes with thrombospondin. Mol Biol Cell. 1992; 3:181–8.

26. Ribeiro SM, Poczatek M, Schultz-Cherry S, Villain M, Murphy-Ullrich JE. The activation sequence of thrombo-spondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factorbeta. J Biol Chem. 1999; 274:13586–93.
27. Good DJ, Plverini PJ, Rastinejad F, Le Beau MM, Lemons RS, Frazier WA, et al. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci USA. 1990; 87:6624–8.

28. Tolsma SS, Volpert OV, Good DJ, Frazier WA. Polverini PJ, Bouck N. Peptides derived from two separate domains of the matrix protein thrombospondin-1 have antiangiogenic activity. J Cell Biol. 1993; 122:497–511.
29. Dawson DW, Bouck NP. Thrombospondin as an inhibitor of angiogenesis. In Antiangiogenic agents in cancer therapy. Edited by Teicher BA. Totowa, NJ: Human Press Inc.;1999. p. 185–203.
30. Kyriakides TR, Leach KJ, Hoffman AS, Ratner BD, Bornstein P. Mice that lack the angiogenesis inhibitor, thrombo-spondin-2, mount an altered foreign body reaction characterized by increased vascularity. Proc Natl Acd Sci USA. 1999; 96:4449–54.
34. Greenwalt DE, Lipsky RH, Ockenhouse CF, Ikeda H, Tandon NN, Jamieson GA. Membrane glycoprotein CD36: A review of its roles in adherence, signal transduction, and transfusion medicine. Blood. 1992; 80:1105–15.

31. Weinstat-Saslow DL, Zabrenetzky VS, VanHoutte K, Frazier WA, Roberts DD, Steeg PS. Transfection of thrombospondin 1 complementary DNA into a human breast carcinoma cell line reduces primary tumor growth, metastatic potential and angiogenesis. Cancer Res. 1994; 54:6504–11.
32. Bleuel K, Popp S, Fusenig NE, Stanbridge EJ, Boukamp P. Tumor suppression in human skin carcinoma cells by chromosome 15 transfer or thrombospondin-1 overexpression through halted tumor vascularization. Proc Natl Acad Sci USA. 1999; 96:2065–70.

35. Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000; 6:41–8.
36. Vogel T, Guo NH, Kruzsch HC, Blake DA, Hartman J, Mendelovitz S, et al. Modulation of endothelial cell proliferation, adhesion, and motility by recombinant heparin-binding domain and synthetic peptides from the type 1 repeats of thrombospondin. J Cell Biochem. 1993; 53:74–84.
37. Kanda S, Shono T, Tomasini-Johansson B, Klint P, Saito Y. Role of thrombospondin-1-derived peptide, 4N1K, in FGF-2-induced angiogenesis. Exp Cell Res. 1999; 252:262–72.

38. Roberts DD, Miller TW, Rogers NM, Yao M, Isenberg JS. The matricellular protein thrombospondin-1 globally regulates cardiovascular function and responses to stress via CD47. Matrix Biol. 2012; 31:162–9.

39. Isenberg JS, Martin-Manso G, Maxhimer JB, Roberts DD. Regulation of nitric oxide signalling by thrombospondin 1: implications for antiangiogenic therapies. Nat Rev Cancer. 2009; 9:182194.

40. Kaur S, Martin-Manso G, Pendrak ML, Garfield SH, Isenberg JS, Roberts DD. Thrombospondin-1 inhibits VEGF re-ceptor-2 signaling by disrupting its association with CD47. J Biol Chem. 2010; 285:38923–32.

41. Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Stare-feldt A, Murphy-Ullrich JE, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through transactivation of LRP on the phagocyte. Cell. 2005; 123:321–34.

42. Chao MP, Jaiswal S, Weissman-Tsukamoto R, Alizadeh AA, Gentles AJ, Volkmer J, et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med. 2010; 2:63ra94.

43. Chao MP, Majeti R, Weissman IL. Programmed cell removal: a new obstacle in the road to developing cancer. Nat Rev Cancer. 2011; 12:58–67.

44. Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000; 288:2051–4.

45. Blazar BR, Lindberg FP, Ingulli E, Panoskaltsis-Mortari A, Oldenborg PA, Iizuka K, et al. CD47 (integrin-associated protein) engagement of dendritic cell and macrophage coun-terreceptors is required to prevent the clearance of donor lymphohematopoietic cells. J Exp Med. 2001; 194:541–9.

46. Barclay AN, Van den Berg TK. The interaction between signal regulatory protein alpha (SIRPα) and CD47: structure, function, and therapeutic target. Annu Rev Immunol. 2014; 32:25–50.

47. Yamao T, Noguchi T, Takeuchi O, Nishiyama U, Morita H, Hagiwara T, et al. Negative regulation of platelet clearance and of the macrophage phagocytic response by the transmembrane glycoprotein SHPS-1. J Biol Chem. 2002; 277:39833–9.

48. Olsson M, Bruhns P, Frazier WA, Ravetch JV, Oldenborg PA. Platelet homeostasis is regulated by platelet expression of CD47 under normal conditions and in passive immune thrombocytopenia. Blood. 2005; 105:3577–82.

49. Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009; 138:271–85.

50. Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD Jr, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009; 138:286–99.

51. Rendtlew Danielsen JM, Knudsen LM, Dahl IM, Lodahl M, Rasmussen T. Dysregulation of CD47 and the ligands thrombospondin 1 and 2 in multiple myeloma. Br J Haematol. 2007; 138:756–60.
52. Chan KS, Espinosa I, Chao M, Wong D, Ailles L, Diehn M, et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumorinitiating cells. Proc Natl Acad Sci USA. 2009; 106:14016–21.

53. Chan KS, Volkmer JP, Weissman I. Cancer stem cells in bladder cancer: a revisited and evolving concept. Curr Opin Urol. 2010; 20:393–7.

54. Liu X, Pu Y, Cron K, Deng L, Kline J, Frazier WA, et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med. 2015; 21:1209–15.

Go to : 
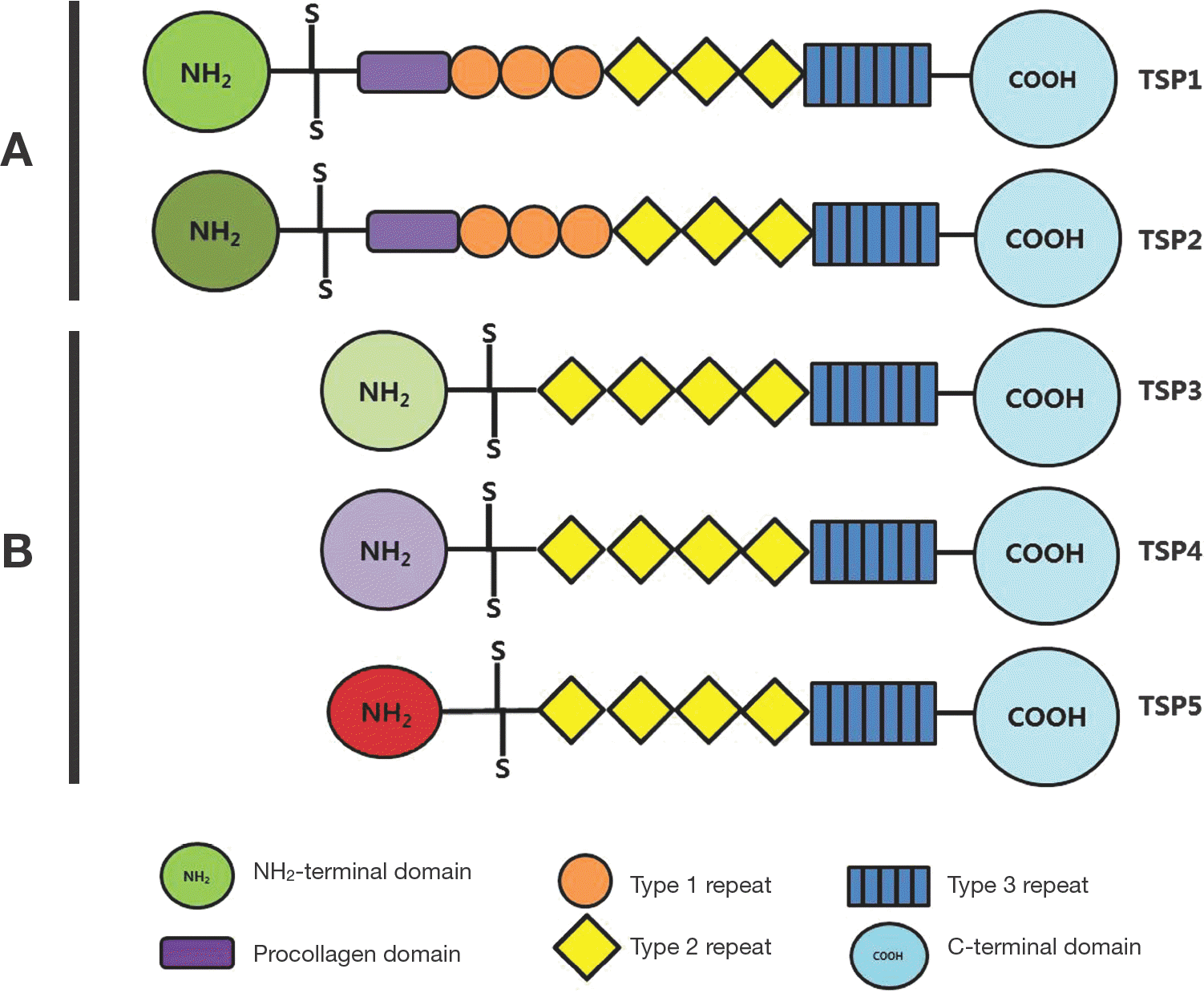 | Fig. 1.Domain structures of human Thrombospondin gene family. The thrombospondins are a family of five extracellular glycoproteins that are composed of multiple well-defined structural motifs. All five members contain the type 2 repeats, the type 3 repeats and a highly conserved C-terminal domain. |
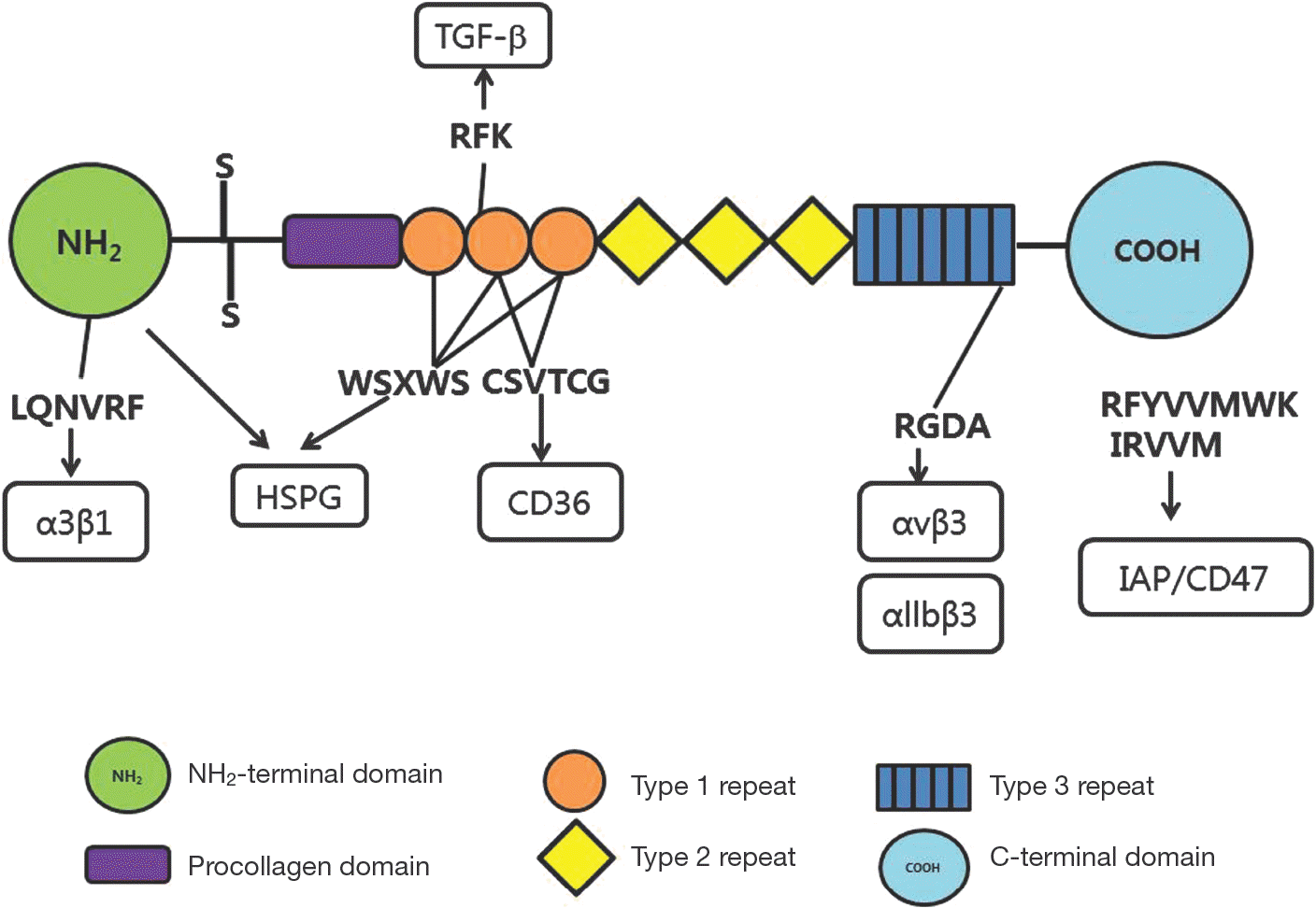 | Fig. 2.The structure of the thrombospondin-1 subunit and its receptors. The TSP-1 peptide consists of six functional domain structures from N- to C-terminal. The NH2-terminal domain stimulates angiogenic response via α3β1 and other integrins. The type 1 repeats contain two distinct anti-angiogenic sequences that interacts with heparan sulfated glycoconjugates, fibronectin, TGF-β, and the receptor CD36. The type 3 repeats contain an RGD sequence that interacts with α5β1 and αvβ3 integrins. The C-terminal domain interacts with CD47. Some CD47-binding peptides derived from this domain inhibit angiogenesis. |
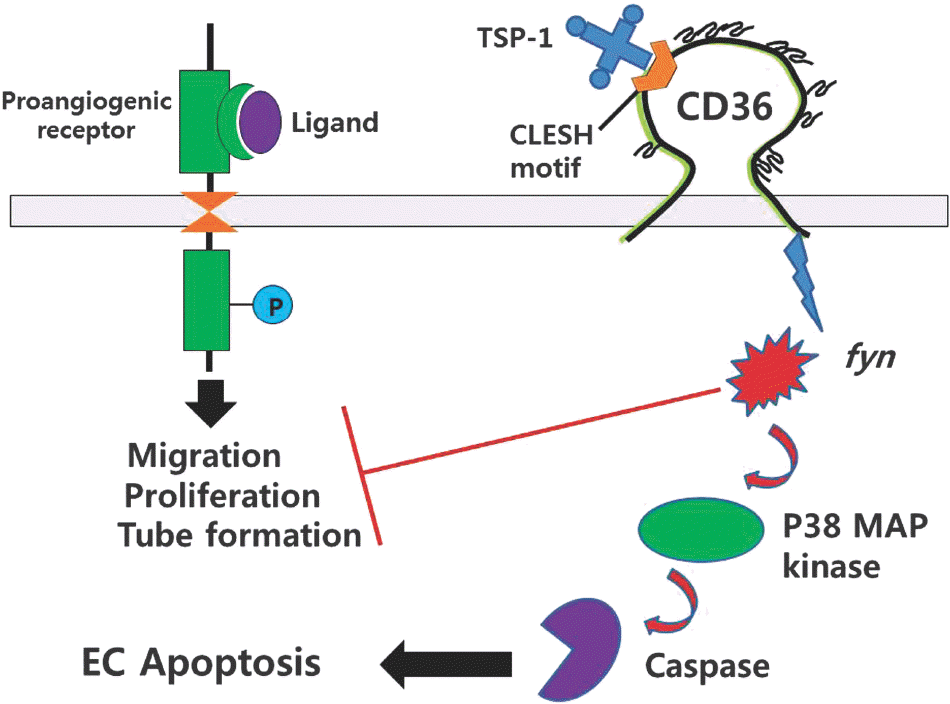 | Fig. 3.Antagonism of angiogenesis by CD36 receptor. Prongio-genic receptors induces proliferation, migraton, and tube formation of endothelial cells by ligand stimulation. In the presence of TSP-1, which interactis with a specific motif[CLESH) of the CD36 receptor, angiogenesis is inhibited. Inhibition is mediated by fyn, p38 MAPK and caspase signaling pathways, resulting in endothe-lial cell apoptosis. |
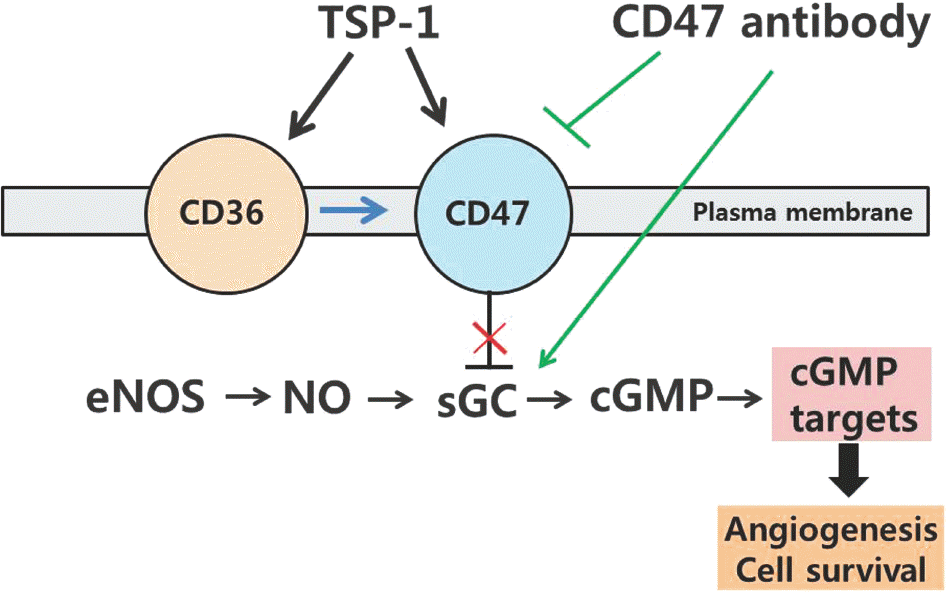 | Fig. 4.TSP1-CD47 signaling pathways. TSP-1 acts through its re-ceptor CD47 to block sGC activation. TSP-1 also signals through CD36 to inhibit the same response, but CD47 is necessary for these signals to inhibit cGMP signaling. Blockade of TSP1-CD47 signaling with antibodies to TSP-1 and CD47 increases cell surviv-al and enhances angiogenesis. |
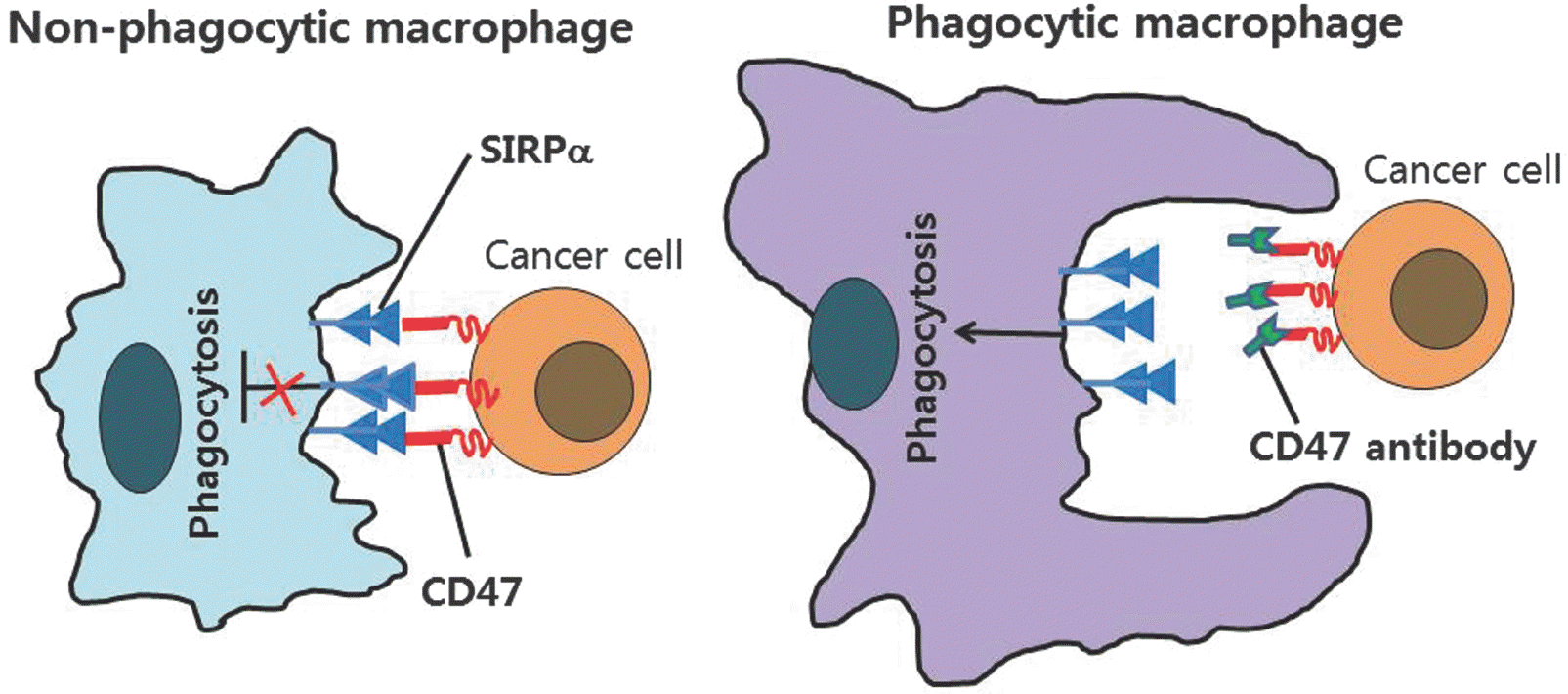 | Fig. 5.Macrophage phagocytosis of tumor cells by CD47 antibody. Left, CD47 expression on cancer cells activates SIRPα on macro-phages and prevents macrophage phagocytosis of cancer cells. Right, CD47-blocking therapies to cancer cells prevent inhibitory signaling from SIRPα to augment macrophage phagocytosis. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download