Abstract
Background
Reducing skin contamination rate and improving the positive rate in blood culture is essential for the correct diagnosis and management of sepsis. Chlorhexidine-alcohol was compared with povidone-iodine for the efficiency of disinfection. Positive rates were compared between the collection of 10 mL and 20 mL of blood per sample.
Methods
The study population included adult patients ≥ 18 years old requested for blood culture in the Emergency Department. Povidone-iodine (10%) was used for antiseptic skin preparation from March to June 2011, and 0.5% chlorhexidine-alcohol from July to October 2011. The standard for blood collection was 10 mL in the first period and 20 mL in the second period. The dedicated phlebotomists had been educated on the optimal skin preparation and sample collection.
Results
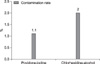
After 10% povidone-iodine application, 31 of 2,755 samples (1.1%) were considered to be contaminated; whereas, a total of 60 of 3,064 samples (2.0%) were contaminated (P=0.011) after application of 0.5% chlorhexidine-alcohol. The positive rate of blood culture was 12.5% (345/2,755) in the first period versus 17.1% (524/3,064) in the second period (P<0.001).
Conclusion
Both disinfectants appeared acceptable for skin preparation for blood culture collection, although chlorhexidine-alcohol had a higher contamination rate than povidone-iodine. The positive rate of blood culture was in accordance with the amount of sample collected. Continuous education and monitoring are needed for the proper collection and management of blood culture.
The contamination of blood cultures is a common problem with important clinical consequences because blood cultures are critical for the diagnosis and management of bloodstream infections. Many blood cultures have produced false-positive results due to the inadequate preparation of the skin for blood collection and contamination of the blood culture may confuse the interpretation of the results. Furthermore, such contamination is related to poor patient care and it may lead to a prolonged hospitalization stay, unnecessary antibiotic use, increased tests, and other healthcare costs [1,2]. The most common contaminants are coagulase-negative staphylococci (CNS). However, these organisms can become significant pathogens in certain cases. Studies found that 25 to 37% of cultures containing CNS showed significant bacteremia [1,3,4]. Another study showed that approximately 50% of the patients with false-positive cultures still received antibiotics although the physicians were quite certain that CNS was contaminant [1]. These findings suggest that there are diagnostic and therapeutic difficulties when these organisms are isolated. The selection of skin antiseptic is a critical step in the prevention of blood culture contamination. We reviewed the effectiveness of different skin antiseptic agents with regard to the rate of false-positive blood culture results. Several studies have revealed that chlorhexidine is superior to povidone-iodine or alcohol alone for skin preparation for blood sample collection [5]. It has been suggested that education of health-care professionals about adequate skin preparation for blood sample collection may be the key to reduce blood culture contamination [6].
The volume of blood drawn for culture is the most important variable in detecting bacteremia or fungemia [7,8]. An inadequate volume (e.g., too little or too much volume) of blood culture prevents pathogens from attaining correct isolation. For adult patients, the recommended volume for blood culture is from 20 to 30 ml for each venipuncture, with the implication that culturing a higher volume of blood containing small numbers of bacteria will improve the recovery of the bacteria [9]. Indeed, the effectiveness of blood cultures increases in accordance with the volume of blood collected [4,8,10]. Because the bacterial number is less than 1 CFU (colony forming unit)/mL in above than half of adulthood bacteremia [11], it is imperative to design a practical blood culture protocol with a large amount of blood volume. However, a multicenter study revealed that the average blood volume for each set was 7.7 mL at 9 university-affiliated hospitals in Korea [12].
The correct use of disinfectants is essential because improper skin preparation is the main cause of contamination. In addition, the sampling of an adequate volume of blood is also mandatory for a successful blood culture. In this study, we compared the efficacy of two skin antiseptics, povidone-iodine and chlorhexidine-alcohol, in preventing blood culture contamination and we further analyzed the efficiency of the increased blood volume recommended by the CLSI guidelines [9].
The study population included adult patients (≥18 years old) requested for blood culture visiting the Emergency Department; the blood samples were obtained percutaneously for culture. The study was conducted between March and October 2011 at Gyeongsang National University Hospital (GNUH) in Korea, an 850 bed tertiary care hospital. Povidone-iodine at 10% was used for antiseptic skin preparation from March to June 2011 (the first period) and 0.5% chlorhexidine-alcohol (in 75% ethyl alcohol) from July to October 2011 (the second period); we compared the efficacy of these two disinfectants. Chlorhexidine-alcohol was prepared from a stock solution by the staff in the Department of Pharmacology. This study was approved by the IRB in our hospital (GNUHIRB-2011-043).
The blood collection was performed by the phlebotomists working in the Emergency Department, neither by physicians nor by nurses. Education regarding the implications of skin antisepsis and the blood volume drawn was provided twice for these personnel during the June-July period (2011). The skin preparation technique included swabbing the area 3 times with the chosen antiseptic, allowing a minimum drying time of 1 minute with 10% povidone-iodine or 20-30 seconds of drying time with 0.5% chlorhexidine-alcohol. The blood specimens for culture were collected by antecubital venipuncture. The standard guideline of blood collection for each set was 10 mL in the first period, whereas it was changed to 20 mL in the second period. The blood collected was evenly divided into aerobic (SA) and anaerobic (SN) bottle. The weight of each set was measured and compared between the two study periods. The blood volume was calculated with the blood density of 1.055 g/mL [10]. During the first period, the phlebotomists were not notified for the measurement of blood volume, whereas they were notified in the second period. Compliance with the study (i.e., the consumption of the assigned antiseptic or obtaining a sufficient blood volume) was monitored and mostly discussed during the second period of the study. However, we did not directly observe the phlebotomy procedures.
The blood was cultured using the BacT/Alert 3D (bioMerieux Inc., Durham, NC, USA.) automated blood culture system. Each blood culture consisted of a set of two (SA Aerobic and SN Anaerobic) bottles. The number of percutaneously drawn cultures performed in the Emergency Department was obtained from the electric medical record (EMR) system and the microbiological data were retrieved by the laboratory information system. The primary end point was the contamination rate of the blood cultures. If a patient had more than one set requested and only one was positive for CNS or viridans group streptococci (VGS), then that culture was regarded contaminated. In cases of Corynebacterium spp., Propionibacterium acnes, Bacillus spp., or Micrococcus spp., all of the cultures from a patient were considered contaminated regardless of the number of bacteria-positive cultures [7]. If a patient had only one culture taken and it was positive for any of these organisms, then that culture was considered contaminated. However, this latter case was very rare because requests for two sets of blood culture per day were made for more than 95% of the patients (data not shown). During each time period, a blood culture was considered positive if organisms grew in one or more than one of the culture bottles. The positive rate was defined as the ratio of positive including the contaminants over all requested number of sets. The positive cultures were reviewed and classified as a true positive or contaminated based on previously described criteria.
Statistical significance was evaluated for the contamination rates between 0.5% chlorhexidine-alcohol and 10% povidone-iodine and for positive rates between 10 mL and 20 mL per set of blood collected by the χ2 test using SPSS, version 17. A P value of <0.05 indicated statistical significance.
The primary analysis was to compare the effectiveness of two different skin antiseptics: 10% povidone-iodine versus 0.5% chlorhexidine-alcohol. A total of 2,755 samples were obtained by venipuncture with disinfection using 10% povidone-iodine from March to June 2011 and 3,064 sets using 0.5% chlorhexidine-alcohol from July to October 2011. With the use of 10% povidone-iodine as the disinfectant, 31 cultures (1.1%) of 2,755 were interpreted as contaminated; whereas, a total of 60 blood specimens (2.0%) were contaminated among 3,064 samples (P=0.011) using 0.5% chlorhexidine-alcohol (Fig. 1). The most common skin contaminants were S. epidermidis (25.8%) and other CNS (41.9%) in the first period; whereas, S. epidermidis (18.3%), other CNS (28.3%), Micrococcus spp. (15%), Propionibacterium acnes (13.3%) and Bacillus spp. (10%) in the second period (data not shown).
The positive rate was 12.5% (345/2,755) with the 10 mL/set collection in the first period versus 17.1% (524/3,064) with the 20 mL/set collection in the second period (P<0.001). In addition, polymicrobial infections (more than one organism) were significantly more common in the second period (6.0%, 28/464) than in the first period (1.9%, 6/314) (P=0.07). The percentages among the true pathogens were as follows: Eschericia coli 28%, CNS 22.3%, Klebsiella pneumoniae 11.8%, Staphylococcus aureus 8.0%, and VGS 6.4% among 314 isolates in the first period versus E. coli 30.4%, CNS 13.2%, K. pneumoniae 11.9%, S. aureus 9.7% and VGS 4.3% among 464 isolates in the second period. The blood culture bottles were arbitrarily chosen and weighed to monitor compliance. The average blood volume of each set for the 227 patients in each period who had 2 requested sets was equally 7.0 mL (SD 2.2 mL and 2.4 mL each) in the first period and 14.9 mL (SD 5.0 mL) and 15.2 mL (SD 4.9 mL) in the second period.
Blood culture is one of the most common laboratory tests implemented for the diagnosis of bloodstream infections. However, the problem of skin contamination has been known to be widespread and the consequences due to contaminated cultures are not negligible. Up to 50% of the positive cultures may be considered contaminated [13] and previous studies have reported that 0.6 to 6.25% of blood cultures were contaminated [1,5]. The proper preparation of the skin prior to obtaining the blood for culture is an imperative step because contaminated blood cultures are problematic to interpret [14]. Some studies found that the costs caused by contaminated cultures were significantly higher due to antibiotic use, total laboratory costs, and microbiology costs than the cost with negative cultures [1,2]. Beyond the financial costs, the suppression of normal flora and the development of antimicrobial resistance due to unnecessary antibiotic usage would be harmful for patients. According to studies reviewing the appropriate skin antiseptic agents to reduce blood culture contamination, no clear evidence suggests the best skin antiseptic agent to prevent false-positive blood culture results [15]. In previous studies, a number of antiseptics have been used for skin preparation for blood cultures, including alcohol, povidone-iodine, tincture of iodine, and chlorhexidine. Povidone-iodine combined with alcohol preparation has been most commonly used in Korea [12], although the other studies have found tincture of iodine to be more effective [2,16]. There are also evidences to suggest less contamination with an alcoholic solution of chlorhexidine compared to povidone-iodine [5,17].
In GNUH, 10% povidone-iodine was routinely used for the preparation of the skin for blood culture until a new protocol was implemented. In the second period, chlorhexidine-alcohol was used instead of povidone-iodine for skin preparation and we found higher rates of contamination when using 0.5% chlorhexidine-alcohol versus 10% povidone-iodine. These results are contrary to the previous findings [5]. More diverse skin contaminants were recovered using chlorhexidine-alcohol, suggesting this disinfectant might be less effective at removing CNS and other skin flora compared to povidone-iodine. However, these results should be interpreted carefully because the study period was not of sufficient duration and the procedure of skin disinfection had not been monitored. Usage of 20 mL syringe which might be more inconvenient than 10 mL syringe, educational intervention before the second period, and short antiseptic drying time of 20-30 seconds might have affected to our results. Alcohol-based disinfectants should be applied by vigorous friction [18]. It is possible that the increased blood volume in the second period might have contributed to the favorable growth of skin contaminants. If the skin contamination rates were already very low, as at our institution, it might have been very difficult to obtain lower incidences. As the recommended contamination rate is ≤3% according to the CLSI guidelines [9], the skin contamination rates with povidone-iodine (1.1%) and chlorhexidine-alcohol (2.0%) seem satisfactory. Because skin contamination in blood culture is critical, this issue should be further studied more in detail. For a more concrete conclusion of the better skin antiseptic, longer study duration, inpatients group, and diverse phlebotomy teams (phlebotomists, physicians, and nurses) with a randomized trial should be designed.
The blood volume is the key parameter for successful blood culture. The positive rates between the 10 mL and 20 mL collection volume per set were significantly different (12.5% versus 17.1%, respectively). However, this result should be carefully interpreted, as the patient population is different between the two periods. Comparing different volumes (such as 10 mL versus 5 mL per bottle) from the same patient should be implemented to exclude these variables. Considering the positive rates recommended by the CLSI guidelines (of 6-12%), the blood culture performance is evaluated as excellent in the Emergency Department at our institution and the phlebotomists followed the new protocol well in terms of the amount of blood collection. However, continuous monitoring and education would be needed for the correct amount of blood volume, because our data showed a little behind the required blood volume (~15 mL for 20 mL/set protocol). We suggest that medical institutions should follow the CLSI guidelines, especially in terms of the optimal sampling amount (20 mL/set), because most of the institutions in Korea currently adopt a 10 mL/set protocol [19]. More than 20 mL/set would not be justifiable, as the broth occupies 40-45 mL of the broth volume and the optimal ratio of blood to broth would be 1 : 5-10 [9]. Interestingly, polymicrobial infections were more common with the 20 mL/set blood collection protocol (6.0% versus 1.9%); however, the conclusion that a higher blood volume collected caused the polymicrobial infections cannot be drawn with our limited data.
Our findings should be interpreted with regard to several limitations. First, the populations studied were different across the time period; the use of different time frames is a potential problem. Second, seasonal factors were not considered; therefore, it is possible that the contamination rates observed were due to changes in the patient populations or seasonal trends. Third, the Emergency Department is a specialized area where emergency situations are common. Although the phlebotomists received education on aseptic techniques twice, this may have been insufficient to change their behaviors satisfactorily. Fourth, this study was performed at a single center during a short period of time; therefore, the results may not be applicable to the other institutions universally. Fifth, there was no wash-out period included and we believe that 1-2 months of a wash-out period after changing the skin disinfectant would be optimal. An analysis of the data for the first 2 months in the second period did not show a remarkable difference from the rest of the period, thus we included the data. Sixth, the time to positive detection was not measured during the study period. Generally, normal skin flora becomes positive after more than 3 to 5 days of incubation; whereas, most of the true pathogens grow within a day [3]. Thus, the measurement of time to positive detection might provide more clues to differentiate the true pathogens from the skin contaminants. The medical records were not reviewed to differentiate the skin contaminants from the true pathogens. Besides, the contamination rate of the blood culture could be different according to the definition of the skin contaminants. Lastly, this is not a randomized, controlled study, which could provide a higher reliability of the results [2,5,15].
In conclusion, both disinfectants seemed acceptable for the preparation of skin for blood culture collection, although chlorhexidine-alcohol had a higher contamination rate than povidone-iodine. The positive rate of blood culture was in accordance with the amount of sample collected. Continuous education and monitoring is needed for the good performance of blood culture.
Figures and Tables
ACKNOWLEDGMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0008757).
References
1. Souvenir D, Anderson DE Jr, Palpant S, Mroch H, Askin S, Anderson J, et al. Blood cultures positive for coagulase-negative staphylococci: antisepsis, pseudobacteremia, and therapy of patients. J Clin Microbiol. 1998. 36:1923–1926.
2. Little JR, Murray PR, Traynor PS, Spitznagel E. A randomized trial of povidone-iodine compared with iodine tincture for venipuncture site disinfection: effects on rates of blood culture contamination. Am J Med. 1999. 107:119–125.
3. Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev. 2006. 19:788–802.
4. Cockerill FR 3rd, Wilson JW, Vetter EA, Goodman KM, Torgerson CA, Harmsen WS, et al. Optimal testing parameters for blood cultures. Clin Infect Dis. 2004. 38:1724–1730.
5. Mimoz O, Karim A, Mercat A, Cosseron M, Falissard B, Parker F, et al. Chlorhexidine compared with povidone-iodine as skin preparation before blood culture. A randomized, controlled trial. Ann Intern Med. 1999. 131:834–837.
6. Eskira S, Gilad J, Schlaeffer P, Hyam E, Peled N, Karakis I, et al. Reduction of blood culture contamination rate by an educational intervention. Clin Microbiol Infect. 2006. 12:818–821.
7. Riedel S, Carroll KC. Blood cultures: key elements for best practices and future directions. J Infect Chemother. 2010. 16:301–316.
8. Li J, Plorde JJ, Carlson LG. Effects of volume and periodicity on blood cultures. J Clin Microbiol. 1994. 32:2829–2831.
9. CLSI. CLSI document M47-A. 2007. Principles and procedures for blood cultures; approved guideline. 2007. Wayne, PA: Clinical and Laboratory Standards Institute.
10. Bouza E, Sousa D, Rodríguez-Créixems M, Lechuz JG, Muñoz P. Is the volume of blood cultured still a significant factor in the diagnosis of bloodstream infections? J Clin Microbiol. 2007. 45:2765–2769.
11. Towns ML, Jarvis WR, Hsueh PR. Guidelines on blood cultures. J Microbiol Immunol Infect. 2010. 43:347–349.
12. Shin JH, Song SA, Kim MN, Lee NY, Kim EC, Kim S, et al. Comprehensive analysis of blood culture performed at nine university hospitals in Korea. Korean J Lab Med. 2011. 31:101–106.
13. Darby JM, Linden P, Pasculle W, Saul M. Utilization and diagnostic yield of blood cultures in a surgical intensive care unit. Crit Care Med. 1997. 25:989–994.
14. Weinstein MP. Blood culture contamination: persisting problems and partial progress. J Clin Microbiol. 2003. 41:2275–2278.
15. Malani A, Trimble K, Parekh V, Chenoweth C, Kaufman S, Saint S. Review of clinical trials of skin antiseptic agents used to reduce blood culture contamination. Infect Control Hosp Epidemiol. 2007. 28:892–895.
16. Schifman RB, Strand CL, Meier FA, Howanitz PJ. Blood culture contamination: a College of American Pathologists Q-Probes study involving 640 institutions and 497134 specimens from adult patients. Arch Pathol Lab Med. 1998. 122:216–221.
17. Shahar E, Wohl-Gottesman BS, Shenkman L. Contamination of blood cultures during venepuncture: fact or myth? Postgrad Med J. 1990. 66:1053–1058.
18. Baron EJ, Weinstein MP, Dunne WM Jr., Yagupsky P, Welch DF, Wilson DM. Baron EJ, editor. Cumitech 1C, blood cultures IV. 2005. Washington, D.C.: ASM Press.
19. Shin JH, Song SA, Kim MN, Kim S. Nationwide survey of blood culture performance regarding skin disinfection, blood collection and laboratory procedures. Korean J Clin Microbiol. 2011. 14:91–96.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download