Abstract
Neisseria flavescens has been rarely reported as a pathogen in the literature. We experienced a case of N. flavescens bacteremia and lung abscess co-infected with Streptococcus sanguis in patient with idiopathic hypereosinophilic syndrome. A 15-year-old boy was diagnosed with idiopathic hypereosinophilic syndrome complicated with pulmonary thromboembolism. He was given systemic steroids and thrombolytics. After 8 weeks of therapy, a lung abscess appeared on the plain chest radiograph. We treated him with empirical antibiotics and carried out surgical drainage. Two types of microorganisms were cultured from both blood and pus samples, obtained in the first day of hospitalization. Pus was aspirated from the lung abscess with an aseptic technique. Neisseria species and S. sanguis were identified using traditional methods. To confirm the identity of the Neisseria species, we conducted further testing using 16S ribosomal ribonucleic acid sequencing whereupon N. flavescens was identified. This is the first case report of pulmonary infection caused by N. flavescens. We suggest that N. flavescens may act as a pathogen.
Neisseria flavescens is one of the non-meningococcal and non-gonococcal Neisseria species that has infrequently caused meningitis, endocarditis, and sepsis [1]. Therefore they are distinguished from meningococcus or gonococcus and so called non-pathogenic Neisseria.
Although N. flavescens can colonize as a normal flora in the upper respiratory tract, an infection of the lower respiratory tract was never reported. However, we experienced a case of a lung abscess caused by N. flavescens with consequential bacteremia.
A 15-year-old boy was transferred to our center with complaints of cough, sputum, and progressive dyspnea for 4 days. The plain chest radiograph showed pneumonic infiltration with pleural effusion in both lungs. The chest CT scan revealed patch consolidation on both lower lobes with pleural effusion and the thromboembolus within the left pulmonary artery. Thromboemboli were also detected in the portal vein and the left common iliac vein on the diagnostic work-up. Laboratory examinations showed a white blood cell count of 23,530/µL, segmental neutrophil of 56%, lymphocyte of 9.9%, eosinophil of 26.3% (6,630/µL), hemoglobin of 13.7 g/dL, platelet count of 77,000/µL, C-reactive protein of 11.4 mg/dL (reference range <0.5 mg/dL) and eosinophil cation protein >200 µg/L (reference range, ≤19 µg/L). He was diagnosed as idiopathic hypereosinophilic syndrome (IHES) complicated with pulmonary thromboembolism [2]. He received systemic steroid therapy for IHES, thrombolytics and anti-coagulation therapy.
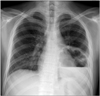
Hypereosinophilia was improved immediately after medication. Methylprednisolone was taken in doses of 2 mg/kg/day for 4 days and then prednisolone was used in doses of 1 mg/kg/day for a month. After 4 weeks of tapering periods, he had intermittent fever and cough over 2 days. The plain chest radiograph showed a shadow of a cavity with an air-fluid level (Fig. 1). He was diagnosed with lung abscess and hospitalized for antibiotics therapy and surgical drainage. We selected the antibiotics nafcillin and cefotaxime empirically.
Bacterial cultures of blood, urine, sputum, and pus drained from the abscess were carried out before antibiotics therapy. Two species of bacteria were cultivated from the blood and the pus obtained at the first day of hospitalization. These two were identified by conventional methods and the Vitek 2 system (BioMérieux, inc., Marcy-l'Etoile, France). One (gram-positive cocci) was identified as Streptococcus sanguis, and the other (gram-negative diplococci) as Neisseria species using Vitek 2 NH Identification Card. To confirm the identity of this isolate, 16S rRNA sequencing analysis was performed. InstaGene Matrix (Bio-Rad Laboratories, Hercules, CA, USA) was used to extract the bacterial genomic DNA, and the first 500 base pairs on the 5' end of the 16S rRNA gene were amplified and sequenced using MicroSeq 500 16S rDNA Bacterial Identification PCR and Sequencing Kits (Applied Biosystems, Foster City, CA, USA). The sequencing product was analyzed on a 3130 Genetic Analyzer (Applied Biosystems) according to the manufacturer's instructions. The resulting sequence was compared with sequences stored in GenBank (http://www.ncbi.nlm.nih.gov/). A GenBank BLAST search revealed that the 16S rRNA gene sequence of the isolate was 100% homologous with that of N. flavescens (GenBank Accession no. GU417548.1). This showed percent identity with >0.8% separation from other species that were listed up in the search (Neisseria gonorrhea, Neisseria weaveri, Neisseria subflava, etc.), and the isolate was confirmed to be N. flavescens [3].
After the antibiotics therapy, the clinical symptoms were improved. According to the results of the bacterial culture, we continued to use cefotaxime except for nafcillin. The sensitivity test of antibiotics was not performed because there was no protocol about the proper sensitivity test for N. flavescens. Subsequently the patient was discharged on the 37th day of hospitalization. On the second month of the follow-up he was in good condition without recurrence.
Neisseria is the genus characteristic of gram-negative diplococci. There are two well-known pathogenic organisms in the genus Neisseria, N. gonorrhea and N. meningitidis. These two organisms characterized the specific disease entity, respectively. N. meningitidis usually colonize in the nasopharynx of humans and sometimes invades into the bloodstream. Moreover, when invaded, it may cause bacteremia, meningitis, and severe meningococcemia. N. gonorrhea infects the genitourinary tract mucosa and rarely causes disseminated infection. While non-pathogenic Neisseria are commensal organisms living in the upper respiratory tract, and relatively few cases of infection were reported compared to pathogenic Neisseria.
Like other non-pathogenic Neisseria, N. flavescens has been rarely reported as a causative microorganism. The first case report of significant infection by this organism was of 14 cases of meningitis, which were a single epidemic in Chicago in 1928 [4]. Subsequently four other cases of meningitis were reported in 1957 [4]. The septicemia by this organism was reported first in 1968 [4]. It was a 20-year-old woman and her symptoms were discovered 7 days after dental surgery. And in 1984, more cases of meningitis and septicemia by N. flavescens were reported, which consisted of 15 cases of meningitis accompanied by septicemia and one case of septicemia [5]. After that, the first case of infectious endocarditis by N. flavescens was noted and reported in 1987 [6]. Infectious endocarditis was also observed in the case of a patient with narcotic addiction and reported in 1990 [7]. A case of septic shock was reported in 1990 which occurred in a pediatric patient [8]. Therefore, our report is one of the rare cases of the infection caused by N. flavescens especially in a pediatric patient, and also the first case report of the lung abscess, which is one of its resultant pulmonary infections.
N. meningitidis causes septicemia, sepsis, and meningitis by invading into the blood stream [9]. In the present case, N. flavescens was found both in the abscess and blood. We predicted that N. flavescens caused localized pulmonary infection and then invaded into the bloodstream through the site.
In this context, the present case demonstrates several characteristic points. First, while, N. flavescens are commensal organisms, yet less virulent than the other pathogenic Neisseria species, it can potentially infect its host.
Second, N. flavescens not only cause sepsis, meningitis or infective endocarditis but it also locally infects the respiratory system. This would be more likely happened in patients who have underlying pulmonary problems or diseases. As with this case, the lung abscess occurred at the site, where pulmonary embolism and combined pneumonia was previously noticed. There are articles supporting this suggestion. One study revealed that the lower respiratory tract was non-sterile in patients with chronic obstructive pulmonary disease (COPD) and the increased number of microbial loads was related to acute exacerbation of COPD [10]. Another study showed common species identified in the lower respiratory tract of COPD patients, which included of Neisseria [11]. In comparison, the low respiratory tract of healthy subjects was relatively sterile [12]. Another study compared patients who had contracted cicatricial laryngeal stenosis after surgery with healthy subjects. The patients showed a significantly greater occurrence of staphylococci, Neisseria and Candida in the pharyngeal mucosa. What is more, they had a higher rate of dysbacteriosis, which was characterized by a predominance of N. flavescens, Staphylococcus aureus, alpha-hemolytic streptococcus, Staphylococcus epidermidis and Candida species [13].
Third, his illness could be influenced by immune suppression following the steroid therapy. The immune deficiency allows an extraordinary infection by a less virulent organism. Abscesses complicated in steroid therapy occur infrequently and have been reported as a case report, but it may be one of the important factors causing the infection.
In interpretating of the organism as a causative micro-organism, it can be argued whether the identified organism is a contaminant or not. In the present case, we believe that N. flavescens was a pathogen co-infected with S. sanguis, because same organisms were cultivated from both blood and pus simultaneously. The specimens were collected by an aseptic technique and the pus was attained by an aspiration through the chest wall not through the airway. The fact that N. flavescens was not a common colonizer of the skin also supports the contention that this microorganism was not a contaminant.
References
1. Feder HM, Garibaldi RA. The significance of nongonococcal, nonmeningococcal Neisseria isolates from blood cultures. Rev Infect Dis. 1984. 6:181–188.
2. Roufosse FE, Goldman M, Cogan E. Hypereosinophilic syndromes. Orphanet J Rare Dis. 2007. 2:37.
3. Clinical and Laboratory Standards Institute. CLSI Document MM18-A. Interpretive criteria for identification of bacteria and fungi by DNA target sequencing: Approved guideline. 2008. Wayne, PA: Clinical and Laboratory Standards Institute.
4. Wertlake PT, Williams TW Jr. Septicaemia caused by Neisseria flavescens. J Clin Pathol. 1968. 21:437–439.
5. Coovadia YM. Neisseria flavescens septicaemia with meningitis. A case report. S Afr Med J. 1984. 66:308–309.
6. Sinave CP, Ratzan KR. Infective endocarditis caused by Neisseria flavescens. Am J Med. 1987. 82:163–164.
7. Szabo S, Lieberman JP, Lue YA. Unusual pathogens in narcoticssociated endocarditis. Rev Infect Dis. 1990. 12:412–415.
8. Quintero Otero S, Rubio Quiñones F, Hernández Gonzalez A, Díaz Portillo J, García Martos P, Pantoja Rosso S. Septic shock caused by Neisseria flavescens. An Esp Pediatr. 1990. 33:64–65.
9. Melican K, Dumenil G. Vascular colonization by Neisseria meningitidis. Curr Opin Microbiol. 2012. 15:50–56.
10. Rosell A, Monsó E, Soler N, Torres F, Angrill J, Riise G, et al. Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Arch Intern Med. 2005. 165:891–897.
11. Cabrera-Rubio R, Garcia-Núñez M, Setó L, Antó JM, Moya A, Monsó E, et al. Microbiome diversity in the bronchial tracts of patients with chronic obstructive pulmonary disease. J Clin Microbiol. 2012. 50:3562–3568.
12. Thorpe JE, Baughman RP, Frame PT, Wesseler TA, Staneck JL. Bronchoalveolar lavage for diagnosing acute bacterial pneumonia. J Infect Dis. 1987. 155:855–861.
13. Kovalyk AP, Govda AV. Characteristics of microflora of laryngeal mucosa in healthy subjects and patients with cicatrical stenosis of the larynx. Vestn Otorinolaringol. 2010. 17–20.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download