Abstract
Background
We evaluated the performance of the AdvanSure MDR-TB GenoBlot Assay kit (AdvanSure MDR-TB, LG Life Science, Korea) to detect mutations related to rifampin (RFP)- and isoniazid (INH)-resistant Mycobacterium tuberculosis complex in respiratory specimens.
Methods
From February 2010 to June 2010, a total of 542 M. tuberculosis clinical isolates were collected from pulmonary tuberculosis patients in six university hospitals across Korea. We analyzed the conventional drug susceptibility testing (DST) and compared the results with those of the AdvanSure MDR-TB.
Results
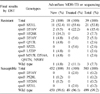
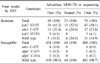
Compared with the conventional DST, the overall agreement rates, sensitivity, and specificity were 98.2% (532/542), 84.6% (33/39), and 99.2% (499/503), respectively, for RFP resistance and 96.1% (521/542), 79.7% (59/74), 98.7% (462/468), respectively, for INH resistance. The three common rpoB mutations were rpoB S531L (53.8%), rpoB D516V (15.4%) and rpoB H526R (7.7%) in RFP-resistant strains. For INH resistance, the katG S315T mutation (58.1%) was the most common, followed by inhA C-15T (23.0%) and katG S315N (4.1%).
Figures and Tables
References
1. Kim BJ, Lee IH, Lee DH, Bai GH, Kong SJ, Lee SH, et al. The current status of multidrug-resistant tuberculosis in Korea. Tuberc Respir Dis. 2006. 60:404–411.
2. Korea Centers for Disease Control and Prevention. Annual report on the notified tuberculosis patients in Korea 2009. 2010. Seoul: Korea Centers for Disease Control and Prevention.
3. World Health Organization. Guidelines for the programmatic management of drug resistant tuberculosis: Emergency update 2008. 2008. Geneva, Switzerland: World Health Organization.
4. Joint Committee for the Development of Korean Guidelines for Tuberculosis. Korean guidelines for tuberculosis. 2011. 1st ed. Chungbuk: Korea Centers for Disease Control and Prevention;32–33.
5. Ahmad S, Mokaddas E. Recent advances in the diagnosis and treatment of multidrug-resistant tuberculosis. Respir Med. 2009. 103:1777–1790.
6. Zhang M, Yue J, Yang YP, Zhang HM, Lei JQ, Jin RL, et al. Detection of mutations associated with isoniazid resistance in Mycobacterium tuberculosis isolates from China. J Clin Microbiol. 2005. 43:5477–5482.
7. Kiepiela P, Bishop KS, Smith AN, Roux L, York DF. Genomic mutations in the katG, inhA and aphC genes are useful for the prediction of isoniazid resistance in Mycobacterium tuberculosis isolates from Kwazulu Natal, South Africa. Tuber Lung Dis. 2000. 80:47–56.
8. Akpaka PE, Baboolal S, Clarke D, Francis L, Rastogi N. Evaluation of methods for rapid detection of resistance to isoniazid and rifampin in Mycobacterium tuberculosis isolates collected in the Caribbean. J Clin Microbiol. 2008. 46:3426–3428.
9. Pai M, Kalantri S, Dheda K. New tools and emerging technologies for the diagnosis of tuberculosis: part II. Active tuberculosis and drug resistance. Expert Rev Mol Diagn. 2006. 6:423–432.
10. Cho EH, Bae HK, Kang SK, Lee EH. Detection of isoniazid and rifampicin resistance by sequencing of katG, inhA, and rpoB genes in Korea. Korean J Lab Med. 2009. 29:455–460.
11. Jeong TD, An D, Sung H, Chi HS, Kim MN, Shim TS. Evaluation of the performance of GenoType® MTBDRplus assay for rapid detection of multi-drug resistant Mycobacterium tuberculosis in sputum specimens. Lab Med Online. 2011. 1:19–25.
12. Espinal MA, Laszlo A, Simonsen L, Boulahbal F, Kim SJ, Reniero A, et al. World Health Organization-International Union against Tuberculosis and Lung Disease Working Group on Anti-Tuberculosis Drug Resistance Surveillance. Global trends in resistance to antituberculosis drugs. N Engl J Med. 2001. 344:1294–1303.
13. Han SB, Jo Y, Yu JK, Kim Y, Park YJ. Performance assessment of AdvanSure™ MDR-TB genoblot assay kit for anti-tuberculosis drug susceptibility test. Lab Med Online. 2012. 2:34–40.
14. Jin J, Zhang Y, Fan X, Diao N, Shao L, Wang F, et al. Evaluation of the GenoType® MTBDRplus assay and identification of a rare mutation for improving MDR-TB detection. Int J Tuberc Lung Dis. 2012. 16:521–526.
15. Telenti A, Imboden P, Marchesi F, Lowrie D, Cole S, Colston MJ, et al. Detection of rifampicin-resistance mutations in Mycobacterium tuberculosis. Lancet. 1993. 341:647–650.
16. Zaczek A, Brzostek A, Augustynowicz-Kopec E, Zwolska Z, Dziadek J. Genetic evaluation of relationship between mutations in rpoB and resistance of Mycobacterium tuberculosis to rifampin. BMC Microbiol. 2009. 9:10.
17. Hazbón MH, Brimacombe M, Bobadilla del Valle M, Cavatore M, Guerrero MI, Varma-Basil M, et al. Population genetics study of isoniazid resistance mutations and evolution of multidrug-resistant Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2006. 50:2640–2649.
18. Kim SY, Park YJ, Kim WI, Lee SH, Ludgerus Chang C, Kang SJ, et al. Molecular analysis of isoniazid resistance in Mycobacterium tuberculosis isolates recovered from South Korea. Diagn Microbiol Infect Dis. 2003. 47:497–502.
19. Abe C, Kobayashi I, Mitarai S, Wada M, Kawabe Y, Takashima T, et al. Biological and molecular characteristics of Mycobacterium tuberculosis clinical isolates with low-level resistance to isoniazid in Japan. J Clin Microbiol. 2008. 46:2263–2268.
20. Rinder H, Mieskes KT, Löscher T. Heteroresistance in Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2001. 5:339–345.
21. Kim BJ, Oh SH, Cho EJ, Park SK. Cross-resistance between rifampicin and rifabutin and its relationship with rpoB gene mutations in clinically isolated MDR-TB strains. Tuberc Respir Dis. 2006. 60:171–179.
22. Lin HH, Kim HY, Yun YJ, Park CG, Kim BJ, Park YG, et al. Mutations of katG and inhA in MDR M. tuberculosis. Tuberc Respir Dis. 2007. 63:128–138.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download