Abstract
Background
Although many laboratories use automated blood culture systems, adequate skin disinfection and optimal blood volume are still critical for successful culture. The authors undertook a nationwide survey to understand the current situation and problems of blood culture in Korea.
Methods
A survey of blood culture was performed in March and April 2010, including disinfectants, blood collection intervals, and recommended blood volumes. The laboratory physicians described the storage condition of culture bottles before delivery to the equipment. For quality control, the positive rate and skin contamination rate were studied.
Results
Replies to the survey were collected from 74 Korean hospitals. Povidone iodine after either isopropyl alcohol or ethanol application was the most common means of skin disinfection. Sampling of a second set of cultures was performed simultaneously in 38% of hospitals and after a 30-min interval in 50%. The recommended blood volume was 10 mL in most cases (69%), but was 20 mL in 24% of cases. The bottles were stored at 37°C before installation in 23% of cases and at room temperature in 16%, whereas 57% were placed directly in the equipment during the night shift. Positive rates ranged 8-10% in 32% of hospitals, 5-8% in 23%, and <5% in 12%. Skin contamination rates were 2-3% in 32% of hospitals, 1-2% in 27%, and >3% in 13%.
Go to : 
REFERENCES
1. Lamy B, Roy P, Carret G, Flandrois JP, Delignette-Muller ML. What is the relevance of obtaining multiple blood samples for culture? A comprehensive model to optimize the strategy for diagnosing bacteremia. Clin Infect Dis. 2002; 35:842–50.

2. Mylotte JM and Tayara A. Blood cultures: clinical aspects and controversies. Eur J Clin Microbiol Infect Dis. 2000; 19:157–63.
3. Clinical and Laboratory Standards Institute. Principles and procedures for blood cultures. Approved guideline. Document M47-A. Wayne, PA;. Clinical and Laboratory Standards Institute;2007.
4. Li J, Plorde JJ, Carlson LG. Effects of volume and periodicity on blood cultures. J Clin Microbiol. 1994; 32:2829–31.

5. Shafazand S and Weinacker AB. Blood cultures in the critical care unit: improving utilization and yield. Chest. 2002; 122:1727–36.
6. Reimer LG, Wilson ML, Weinstein MP. Update on detection of bacteremia and fungemia. Clin Microbiol Rev. 1997; 10:444–65.

7. Cockerill FR 3rd, Wilson JW, Vetter EA, Goodman KM, Torgerson CA, Harmsen WS, et al. Optimal testing parameters for blood cultures. Clin Infect Dis. 2004; 38:1724–30.

8. Chandrasekar PH and Brown WJ. Clinical issues of blood cultures. Arch Intern Med. 1994; 154:841–9.

9. Lemming L, Holt HM, Petersen IS, Østergaard C, Bruun B. Bactec 9240 blood culture system: to preincubate at 35 degrees C or not? Clin Microbiol Infect. 2004; 10:1089–91.
10. Riedel S and Carroll KC. Blood cultures: key elements for best practices and future directions. J Infect Chemother. 2010; 16:301–16.
11. Linder N, Davidovitch N, Reichman B, Kuint J, Lubin D, Meyerovitch J, et al. Topical iodine-containing antiseptics and subclinical hypothyroidism in preterm infants. J Pediatr. 1997; 131:434–9.

12. Calfee DP and Farr BM. Comparison of four antiseptic preparations for skin in the prevention of contamination of percutaneously drawn blood cultures: a randomized trial. J Clin Microbiol. 2002; 40:1660–5.

13. Barenfanger J, Drake C, Lawhorn J, Verhulst SJ. Comparison of chlorhexidine and tincture of iodine for skin antisepsis in preparation for blood sample collection. J Clin Microbiol. 2004; 42:2216–7.

14. Malani A, Trimble K, Parekh V, Chenoweth C, Kaufman S, Saint S. Review of clinical trials of skin antiseptic agents used to reduce blood culture contamination. Infect Control Hosp Epidemiol. 2007; 28:892–5.

15. Hall KK and Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev. 2006; 19:788–802.

16. Panlilio AL, Beck-Sague CM, Siegel JD, Anderson RL, Yetts SY, Clark NC, et al. Infections and pseudoinfections due to povidone-iodine solution contaminated with Pseudomonas cepacia. Clin Infect Dis. 1992; 14:1078–83.

17. Towns ML, Jarvis WR, Hsueh PR. Guidelines on blood cultures. J Microbiol Immunol Infect. 2010; 43:347–9.

Go to : 
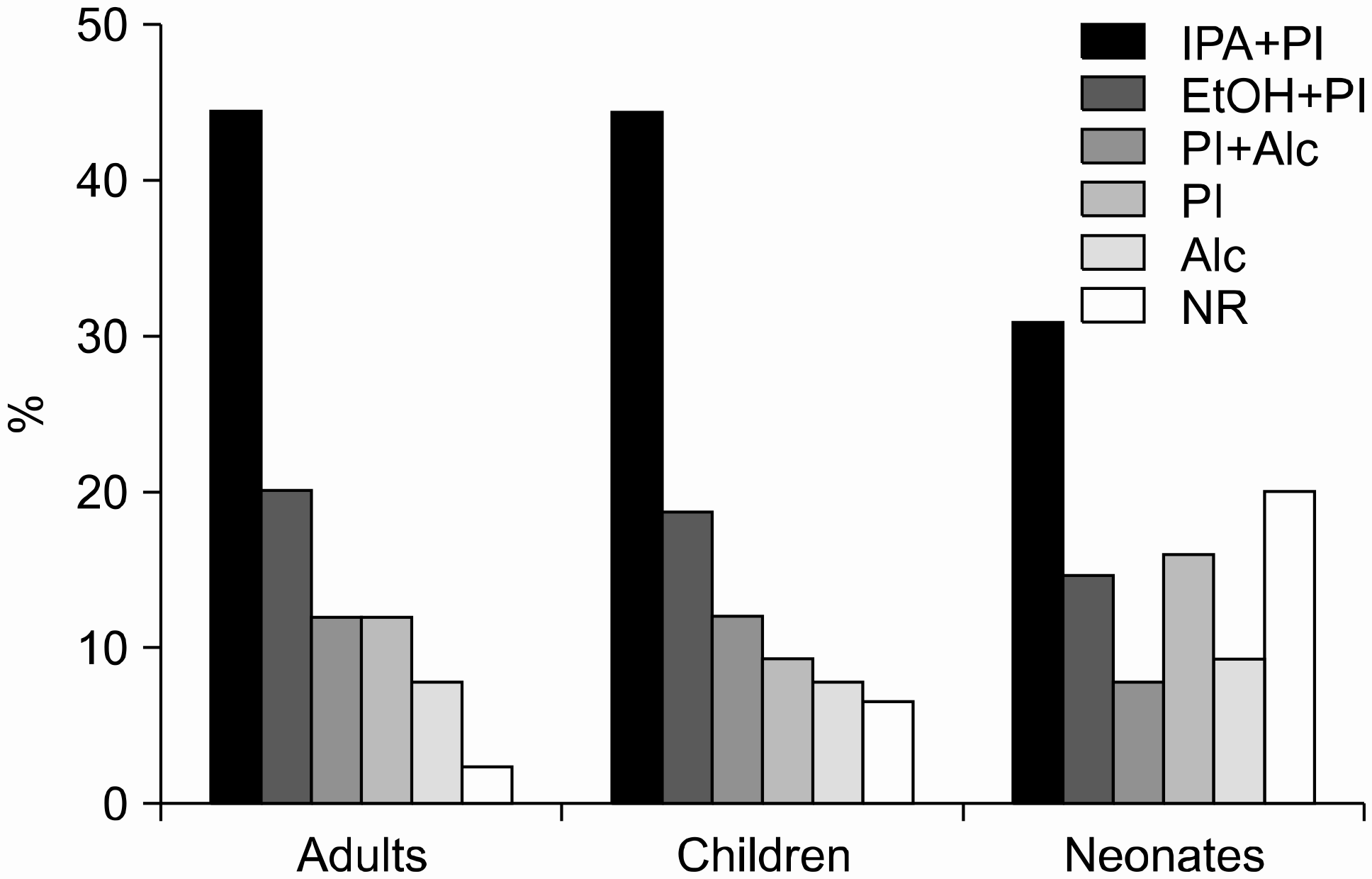 | Fig. 1.Disinfectants for the skin preparation of blood culture. IPA, isopropyl alcohol; PI, povidone iodine; EtOH, ethanol; Alc, isopropyl alcohol or ethanol; NR, no response. |
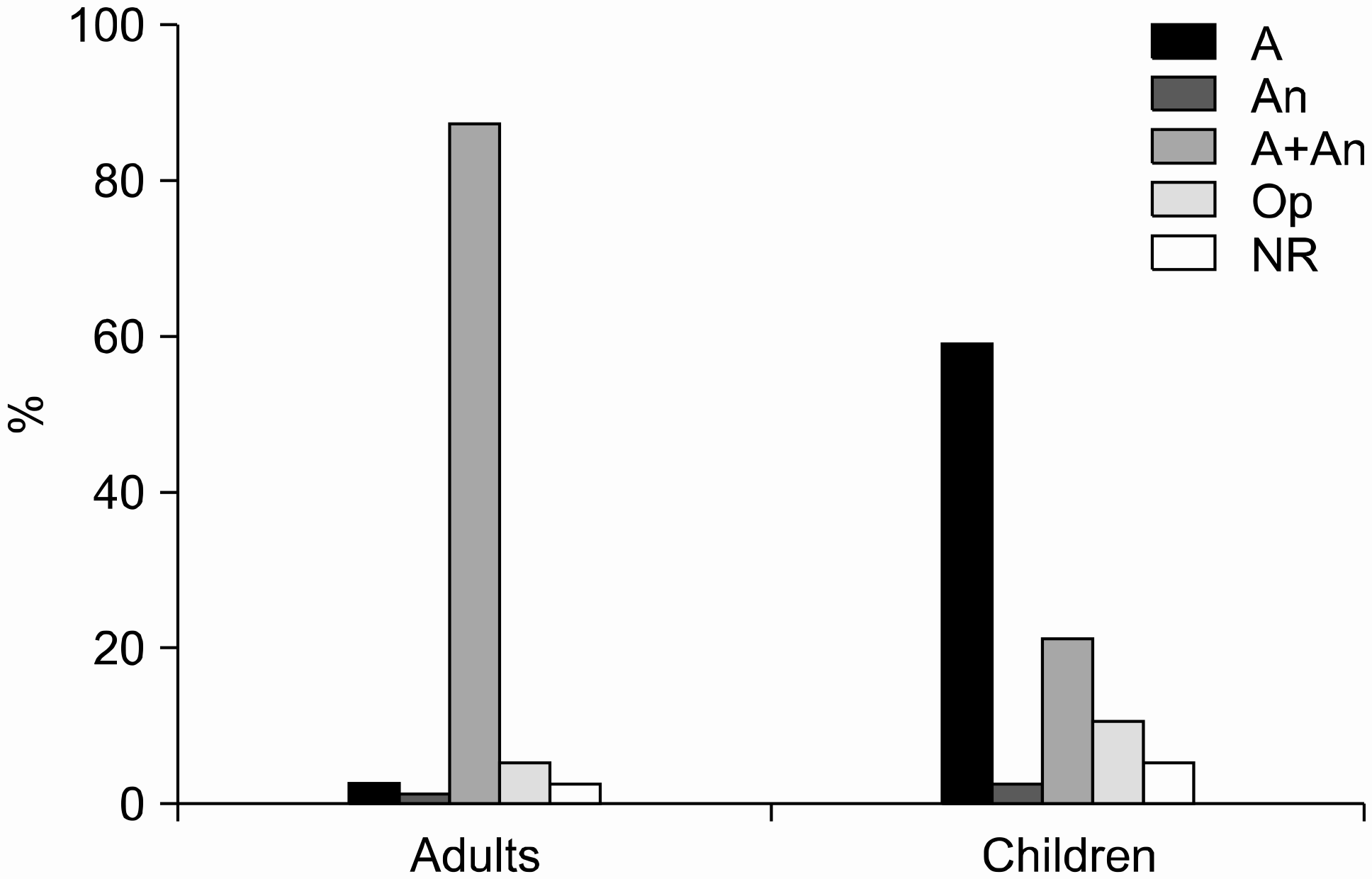 | Fig. 2.Use of blood culture bottles. A, aerobic; An, anaerobic; Op, optional anaerobic; NR, no response. |
Table 1.
Positive rate and contamination rate of blood cultures in 74 hospitals




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download