Abstract
Background
Group A Streptococcus (GAS) is responsible for a wide spectrum of human diseases. We investigated the distribution of emm types and antibiotic resistance rates of GAS from clinical specimens in several Korean medical centers.
Methods
A total of 192 strains of GAS from throat, blood, and other specimens collected in Seoul, Busan, Ulsan, Iksan, and Jeju were studied in 2008-2009. The emm genotypes were identified using PCR and sequencing. Antimicrobial susceptibility testing was performed by disk diffusion method. Phenotypes of macrolide resistance were evaluated, and macrolide resistance genes were determined by PCR.
Results
The emm89 (33.9%) was most frequently detected, followed by emm1 (12.5%), emm12 (8.3%), emm4 (7.8%), and emm75 (7.3%). The distribution of emm types did not show a close relation to the type of specimen and was different for each area. The resistance rates to erythromycin (ERY) and clindamycin (CLI) were 4.6% and 3.7%, respectively. Among the nine ERY-resistant strains, the rate of constitutive resistance was 88.9%, compared with 11.1% for the M phenotype. Five of the ERY-resistant strains were emm28.
Go to : 
REFERENCES
1. Eriksson BK, Norgren M, McGregor K, Spratt BG, Normark BH. Group A streptococcal infections in Sweden: a comparative study of invasive and noninvasive infections and analysis of dominant T28 emm28 isolates. Clin Infect Dis. 2003; 37:1189–93.

2. Shulman ST, Tanz RR, Dale JB, Beall B, Kabat W, Kabat K, et al. North American Streptococcal Pharyngitis Surveillance Group. Seven-year surveillance of north american pediatric group a streptococcal pharyngitis isolates. Clin Infect Dis. 2009; 49:78–84.

3. Beall B, Facklam R, Thompson T. Sequencing emm-specific PCR products for routine and accurate typing of group A streptococci. J Clin Microbiol. 1996; 34:953–8.

4. Choi EH. Emergence of macrolide resistance and clinical use of macrolide antimicrobials in children. Korean J Pediatr. 2008; 51:1031–7.

5. Kim S and Lee NY. Antibiotic resistance and genotypic characteristics of group a streptococci associated with acute pharyngitis in Korea. Microb Drug Resist. 2004; 10:300–5.
6. Yi YH, Choi JH, Lee HK, Lee KJ, Bae SM, Yu JY, et al. Characterization of erythromycin resistance of Streptococcus pyogenes isolated from pharyngitis patients in Korea. Jpn J Infect Dis. 2006; 59:192–4.
7. Chang H, Shen X, Fu Z, Liu L, Shen Y, Liu X, et al. Antibiotic resistance and molecular analysis of Streptococcus pyogenes isolated from healthy schoolchildren in China. Scand J Infect Dis. 2010; 42:84–9.

8. Katz KC, McGeer AJ, Duncan CL, Ashi-Sulaiman A, Willey BM, Sarabia A, et al. Emergence of macrolide resistance in throat culture isolates of group a streptococci in Ontario, Canada, in 2001. Antimicrob Agents Chemother. 2003; 47:2370–2.

9. Pérez-Trallero E, Montes M, Orden B, Tamayo E, García-Arenzana JM, Marimón JM. Phenotypic and genotypic characterization of Streptococcus pyogenes isolates displaying the MLSB phenotype of macrolide resistance in Spain, 1999 to 2005. Antimicrob Agents Chemother. 2007; 51:1228–33.
10. Murakami J, Kawabata S, Terao Y, Kikuchi K, Totsuka K, Tamaru A, et al. Distribution of emm genotypes and superantigen genes of Streptococcus pyogenes isolated in Japan, 1994-9. Epidemiol Infect. 2002; 128:397–404.

11. Lee SY, Lee JS, Lee MA, Chung WS, Kim SJ. Streptococcal toxic shock syndrome associated with intrauterine fetal death: a case report. Korean J Clin Microbiol. 1998; 1:109–12.
12. Chan JC, Chu YW, Chu MY, Cheung TK, Lo JY. Epidemiological analysis of Streptococcus pyogenes infections in Hong Kong. Pathology. 2009; 41:681–6.

13. McNeil SA, Halperin SA, Langley JM, Smith B, Warren A, Sharratt GP, et al. Safety and immunogenicity of 26-valent group a streptococcus vaccine in healthy adult volunteers. Clin Infect Dis. 2005; 41:1114–22.

14. Koh E and Kim S. Decline in erythromycin resistance in group a streptococci from acute pharyngitis due to changes in the emm genotypes rather than restriction of antibiotic use. Korean J Lab Med. 2010; 30:485–90.
15. Oliver MA, García-Delafuente C, Cano ME, Pérez-Hernández F, Martínez-Martínez L, Albertí S. Rapid decrease in the prevalence of macrolide-resistant group A streptococci due to the appearance of two epidemic clones in Cantabria (Spain). J Antimicrob Chemother. 2007; 60:450–2.

16. Ardanuy C, Domenech A, Rolo D, Calatayud L, Tubau F, Ayats J, et al. Molecular characterization of macrolide- and multidrug-resistant Streptococcus pyogenes isolated from adult patients in Barcelona, Spain (1993-2008). J Antimicrob Chemother. 2010; 65:634–43.

17. Seppälä H, Klaukka T, Vuopio-Varkila J, Muotiala A, Helenius H, Lager K, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N Engl J Med. 1997; 337:441–6.
18. Bass JW, Weisse ME, Plymyer MR, Murphy S, Eberly BJ. Decline of erythromycin resistance of group A beta-hemolytic streptococci in Japan. Comparison with worldwide reports. Arch Pediatr Adolesc Med. 1994; 148:67–71.
Go to : 
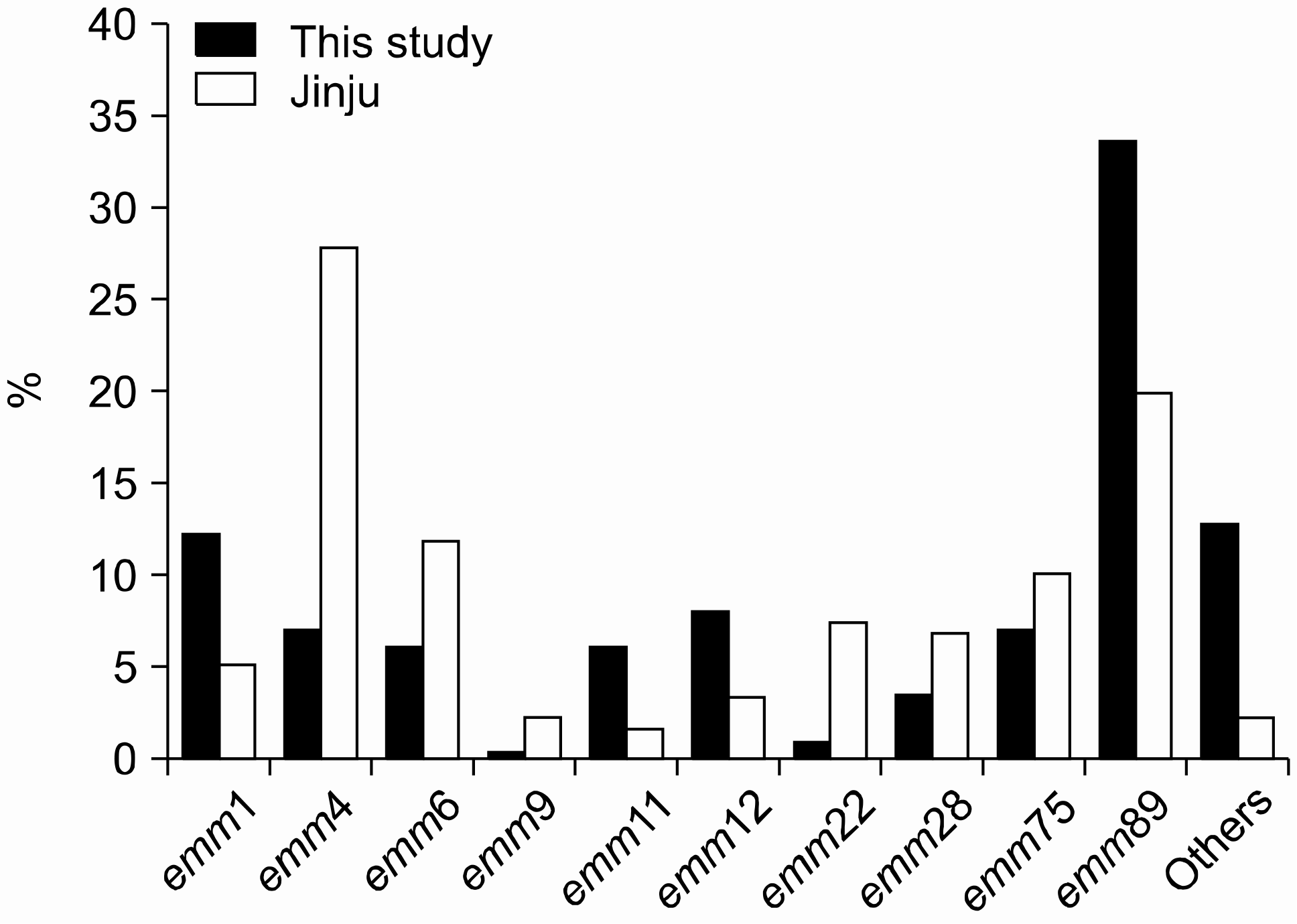 | Fig. 1.Frequency of emm genotypes in this study and study of acute pharyngitis in Jinju, 2009 [14]. |
Table 1.
Distribution of emm genotypes according to specimen type
Table 2.
Distribution of emm genotypes according to geographic region
Table 3.
Minimal inhibitory concentrations (MIC) of erythromycin (ERY) and macrolide-resistant determinants among ERY-resistant group A streptococci




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download