Abstract
Background
Blood culture is important for determining the etiologic agents of bacteremia and fungemia. Analyses of blood culture results and antimicrobial susceptibility can provide clinicians with relevant information for the empirical treatment of patients. The present study was conducted to assess the frequencies and antimicrobial resistance patterns of clinically important microorganisms from nine hospitals.
Methods
Data including microbiological isolates and corresponding antimicrobial susceptibility test results were collected during 2009 from nine and five university hospitals, respectively. Microorganism identification was based on conventional methods. Antimicrobial susceptibility was tested using the VITEK II system or the Clinical and Laboratory Standards Institute disk diffusion method.
Results
Of 397,602 blood specimens cultured from nine hospitals, 34,708 (8.7%) were positive for microorganisms. Excluding coagulase-negative Staphylococci (CoNS), Escherichia coli was the most common isolate (13.5%), followed by Staphylococcus aureus (11.5%), Klebsiella pneumoniae (6.5%) and Enterococcus faecium (3.4%). The isolation rate of CoNS was 23.6%, while that of ceftazidime-resistant E. coli showed geographic differences ranging from 11% to 28%. Among the Gram-negative isolates, A. baumannii displayed the highest levels of resistance. The total isolation rate of the Candida species increased compared to the previous reported rate in Korea.
Go to : 
REFERENCES
1. National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004; 32:470–85.
2. Edmond MB, Wallace SE, McClish DK, Pfaller MA, Jones RN, Wenzel RP. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin Infect Dis. 1999; 29:239–44.

3. Diekema DJ, Pfaller MA, Jones RN. SENTRY Participants Group. Age-related trends in pathogen frequency and antimicrobial susceptibility of bloodstream isolates in North America: SENTRY Antimicrobial Surveillance Program, 1997∼2000. Int J Antimicrob Agents. 2002; 20:412–8.
4. Smith TL and Jarvis WR. Antimicrobial resistance in Staphylococcus aureus. Microbes Infect. 1999; 1:795–805.
5. Diekema DJ, Pfaller MA, Schmitz FJ, Smayevsky J, Bell J, Jones RN, et al. SENTRY Partcipants Group. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin Infect Dis. 2001; 32(Suppl 2):S114–32.
6. Doern GV, Jones RN, Pfaller MA, Erwin M, Ramirez-Rhonda C. Multicenter evaluation of the in vitro activity of six broad-spectrum beta-lactam antimicrobial agents in Puerto Rico. The Puerto Rico Antimicrobial Resistance Study Group. Diagn Microbiol Infect Dis. 1998; 30:113–9.
7. Diekema DJ, Pfaller MA, Jones RN, Doern GV, Winokur PL, Gales AC, et al. Survey of bloodstream infections due to gram-negative bacilli: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, and Latin America for the SENTRY Antimicrobial Surveillance Program, 1997. Clin Infect Dis. 1999; 29:595–607.

8. Heinemann B, Wisplinghoff H, Edmond M, Seifert H. Comparative activities of ciprofloxacin, clinafloxacin, gatifloxacin, gemifloxacin, levofloxacin, moxifloxacin, and trovafloxacin against epidemiologically defined Acinetobacter baumannii strains. Antimicrob Agents Chemother. 2000; 44:2211–3.
9. Diekema DJ, Messer SA, Brueggemann AB, Coffman SL, Doern GV, Herwaldt LA, et al. Epidemiology of candidemia: 3-year results from the emerging infections and the epidemiology of Iowa organisms study. J Clin Microbiol. 2002; 40:1298–302.

10. Hajjeh RA, Sofair AN, Harrison LH, Lyon GM, Arthington-Skaggs BA, Mirza SA, et al. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J Clin Microbiol. 2004; 42:1519–27.
11. National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing. Twenlfth informational supplement, M100-S20. Wayne, PA: National Committee for Clinical Laboratory Standards.;2010.
12. Koh EM, Lee SG, Kim CK, Kim M, Yong D, Lee K, et al. Microorganisms isolated from blood cultures and their antimicrobial susceptibility patterns at a university hospital during 1994∼2003. Korean J Lab Med. 2007; 27:265–75.

13. Kang SH and Kim YR. Characteristics of microorganisms isolated from blood cultures at a university hospital located in an island region during 2003∼2007. Korean J Clin Microbiol. 2008; 11:11–7.

14. Ahn GY, Jang SJ, Lee SH, Jeong OY, Chaulagain BP, Moon DS, et al. Trends of the species and antimicrobial susceptibility of microorganisms isolated from blood cultures of patients. Korean J Clin Microbiol. 2006; 9:42–50.
15. Cockerill FR 3rd, Wilson JW, Vetter EA, Goodman KM, Torgerson CA, Harmsen WS, et al. Optimal testing parameters for blood cultures. Clin Infect Dis. 2004; 38:1724–30.

16. Hall KK and Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev. 2006; 19:788–802.

17. Bates DW, Goldman L, Lee TH. Contaminant blood cultures and resource utilization. The true consequences of false-positive results. JAMA. 1991; 265:365–9.

18. Shafazand S and Weinacker AB. Blood cultures in the critical care unit: improving utilization and yield. Chest. 2002; 122:1727–36.
19. Souvenir D, Anderson DE Jr, Palpant S, Mroch H, Askin S, Anderson J, et al. Blood cultures positive for coagulase-negative staphylococci: antisepsis, pseudobacteremia, and therapy of patients. J Clin Microbiol. 1998; 36:1923–6.

21. Lee JS, Shin JH, Lee K, Kim MN, Shin BM, Uh Y, et al. Species distribution and susceptibility to azole antifungals of Candida bloodstream isolates from eight university hospitals in Korea. Yonsei Med J. 2007; 48:779–86.

22. Weinstein MP, Towns ML, Quartey SM, Mirrett S, Reimer LG, Parmigiani G, et al. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997; 24:584–602.

23. Kim SY, Lim G, Kim MJ, Suh JT, Lee HJ. Trends in five-year blood cultures of patients at a university hospital (2003∼2007). Korean J Clin Microbiol. 2009; 12:163–8.

24. Lee K, Lim CH, Cho JH, Lee WG, Uh Y, Kim HJ, et al. KONSAR Group. High prevalence of ceftazidime-resistant Klebsiella pneumoniae and increase of imipenem-resistant Pseudomonas aeruginosa and Acinetobacter spp. in Korea: a KONSAR program in 2004. Yonsei Med J. 2006; 47:634–45.

25. Song JH, Jung SI, Ko KS, Kim NY, Son JS, Chang HH, et al. High prevalence of antimicrobial resistance among clinical Streptococcus pneumoniae isolates in Asia (an ANSORP study). Antimicrob Agents Chemother. 2004; 48:2101–7.
26. Kim KH, Kim JE, Park SH, Song YH, Ahn JY, Park PW, et al. Impact of revised penicillin breakpoints for Streptococcus pneumoniae (CLSI M100-S18) on the penicillin susceptibility rate. Korean J Clin Microbiol. 2010; 13:68–72.
27. Centers for Disease Control and Prevention (CDC). Effects of new penicillin susceptibility breakpoints for Streptococcus pneumoniae–United States, 2006∼2007. MMWR Morb Mortal Wkly Rep. 2008; 57:1353–5.
28. Hong SG, Kim S, Jeong SH, Chang CL, Cho SR, Ahn JY, et al. Prevalence & diversity of extended-spectrum beta-lactamase- producing Escherichia coli and Klebsiella pneumoniae isolates in Korea. Korean J Clin Microbiol. 2003; 6:149–55.
Go to : 
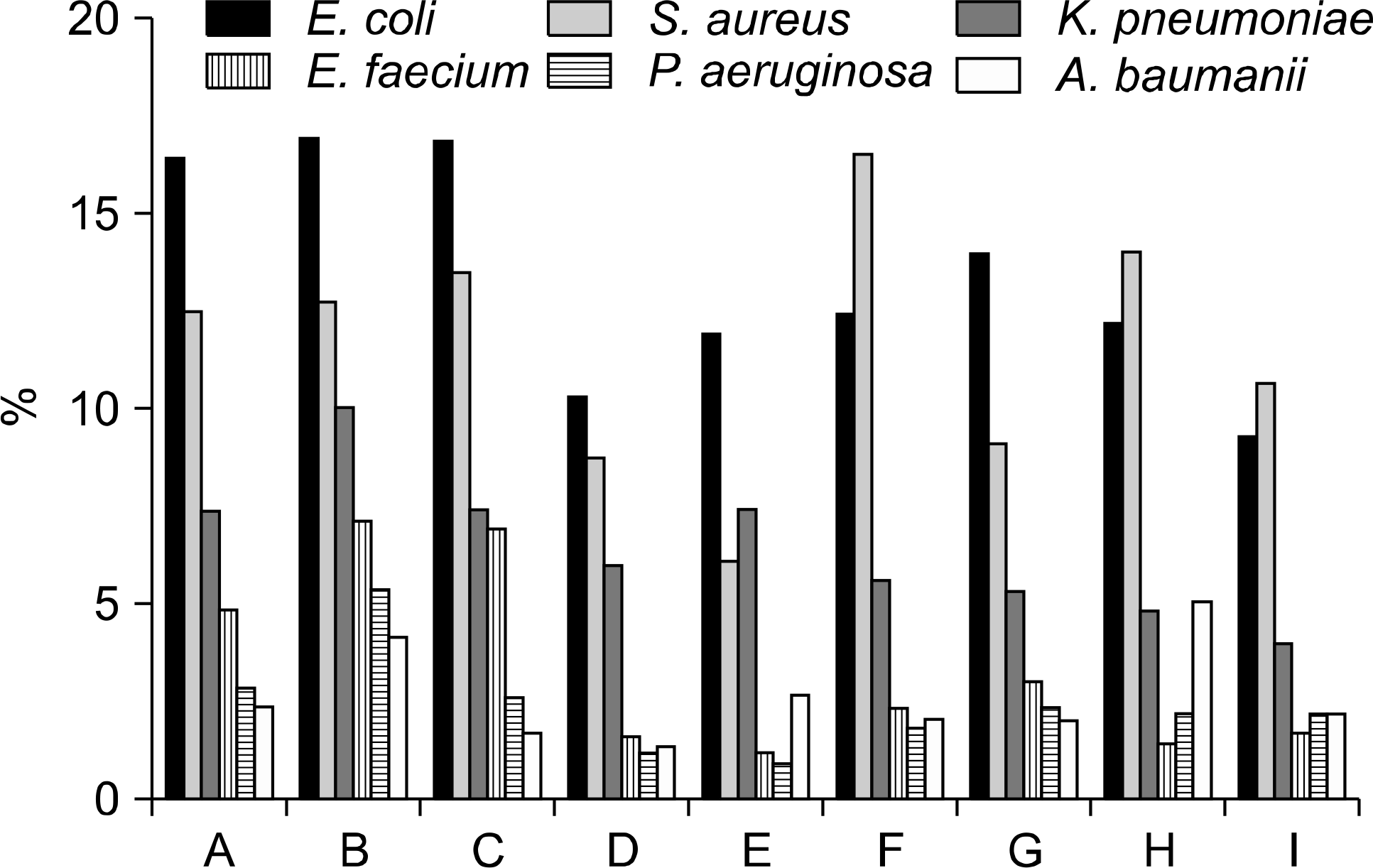 | Fig. 1.The isolation frequencies of common bacteria from 9 hospitals. A, Asan Medical Center; B, Samsung Medical Center; C, Seoul National University Hospital; D, Gyeongsang National University Hospital; E, Inje University Paik Hospital; F, Chungnam National University Hospital; G, Dongsan Medical Hospital; H, Wonkwang University Hospital; I, Kangdong Sacred Heart Hospital. |
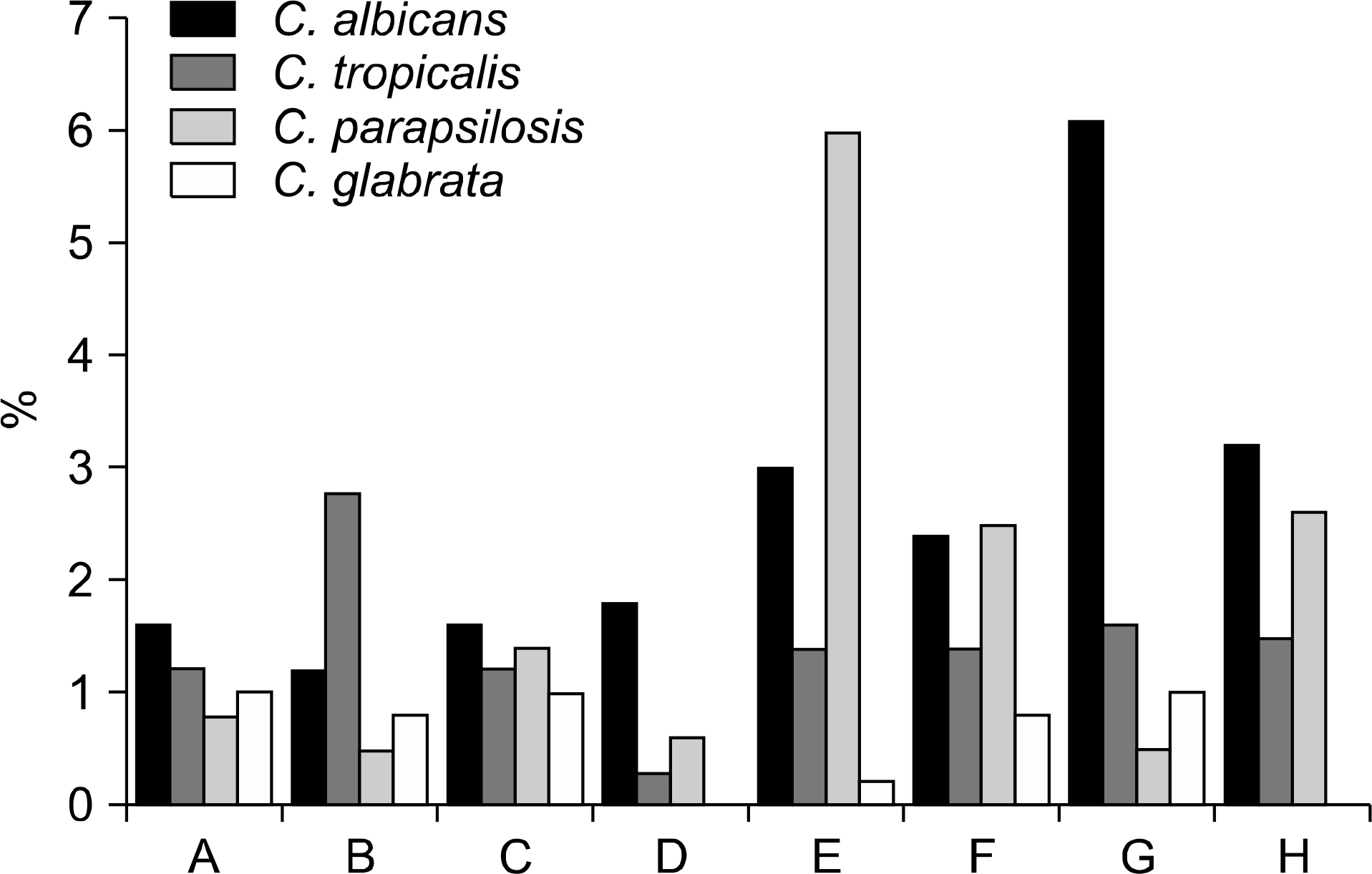 | Fig. 2.The isolation frequencies of common yeasts from 9 hospitals. See Fig. 2 for designations for each hospital (A∼H). |
Table 1.
Rates of antibiotic resistance among the most common Gram-positive bacteria causing bloodstream infections from 5 Korean hospitals in 2009
| Staphylococcus aureus | Staphylococcus epidermidis | Enterococcus faecium | Enterococcus faecalis | Streptococcus pneumoniae | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % resistance | N | % resistance | N | % resistance | N | % resistance | N | % resistance | |
| Ampicillin | ND | NA | ND | NA | 336 | 91.2 | 186 | 14.2 | ND | NA |
| Penicillin | 1121 | 95.4 | 1032 | 96.8 | 336 | 92.1 | 186 | 15.7 | 49 | 20.9 |
| Clindamycin | 1121 | 63.8 | 1032 | 65.9 | ND | NA | ND | NA | ND | NA |
| Oxacillin | 1072 | 69.8 | 1032 | 84.7 | ND | NA | ND | NA | ND | NA |
| QNP-DFP | 1072 | 0.2 | 1032 | 0.1 | ND | NA | ND | NA | ND | NA |
| Cefotaxime∗ | ND | NA | ND | NA | ND | NA | ND | NA | 52 | 7.3 |
| Cefotaxime† | ND | NA | ND | NA | ND | NA | ND | NA | 52 | 5.4 |
| Erythromycin | 1121 | 61.0 | 1032 | 71.3 | ND | NA | ND | NA | 59 | 61.5 |
| Ciprofloxacin | 1121 | 56.4 | 1032 | 58.8 | ND | NA | ND | NA | ND | NA |
| Levofloxacin | 731 | 62.4 | 656 | 26.2 | ND | NA | ND | NA | 59 | 7.9 |
| Gentamicin | 947 | 61.4 | 1032 | 63.7 | ND | NA | ND | NA | ND | NA |
| HL Gentamicin | ND | NA | ND | NA | 325 | 49.2 | 186 | 37.9 | ND | NA |
| HL Streptomycin | ND | NA | ND | NA | 325 | 8.0 | 186 | 16.9 | ND | NA |
| Rifampin | 502 | 2.8 | 1032 | 16.2 | ND | NA | ND | NA | ND | NA |
| Tetracycline | 1121 | 55.8 | 1032 | 22.9 | ND | NA | ND | NA | 59 | 68.1 |
| TMP-SMX | 502 | 7.3 | 522 | 49.7 | ND | NA | ND | NA | 34 | 24.6 |
| Vancomycin | 1072 | 0.0 | 1032 | 0.0 | 336 | 28.6 | 186 | 0.0 | 52 | 0.0 |
Table 2.
Rates of antibiotic resistance among the most common Gram-negative rods causing bloodstream infections from 5 Korean hospitals in 2009
Table 3.
Rank order of microorganisms or microorganism groups most frequently isolated from blood samples
Abbreviations: CoNS, coagulase-negative staphylococci; S. aureus, Staphylococcus aureus; E. coli, Escherichia coli; K. pneumoniae, Klebsiella pneumoniae; S. maltophilia, Stenotrophomonas maltophilia; SVG, Streptococcus viridans group; E. faecium, Enterococcus faecium; S. marcescens, Serratia marcescens; C. albicans, Candida albicans.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download