Abstract
Molecular strain typing of Mycobacterium tuberculosis is important for the detection of outbreaks of tuberculosis and laboratory cross contamination, as well as the differentiation between re-infection and reactivation of tuberculosis. In the present review, the authors investigated the currently available typing methods for M. tuberculosis and the current status of strain distribution in Korea. IS6110-restriction fragment length polymorphism (RFLP), which is considered a standard method, is based on numbers and positions of the insertion sequence, IS6110. The method has an excellent discriminatory power with a considerable amount of worldwide data, although it is time-consuming and labor-intensive. Spoligotyping is based on the presence or absence of spacer sequences between direct repeat (DR) regions. PCR amplification allows for the possibility of application in the early suspicious stage. The data can be easily digitized; however, it shows identical profiles in Beijing family strains. Mycobacterial interspersed repetitive unit-variable number of tandem repeat (MIRU-VNTR) is another PCR-based genotyping method with a good discrimination power whose data can also be easily digitized. In Korea, the prevalence of Beijing family strains have been as high as 80 to 87%.
Go to : 
REFERENCES
1. Hong YP, Kim SJ, Lew WJ, Lee EK, Han YC. The seventh nationwide tuberculosis prevalence survey in Korea, 1995. Int J Tuberc Lung Dis. 1998; 2:27–36.
2. World Health Organization. Tuberculosis control in the Western Pacific region: 2008 Report;. 2008. http://www.medcastle.com/tuberculosis-control-in-the-western-pacific-region-2008-report.html.
3. Aristimuño L, Armengol R, Cebollada A, España M, Guilarte A, Lafoz C, et al. Molecular characterisation of Mycobacterium tuberculosis isolates in the First National Survey of Anti-tuberculosis Drug Resistance from Venezuela. BMC Microbiol. 2006; 6:90.

4. Kim SJ, Bai GH, Lee H, Kim HJ, Lew WJ, Park YK, et al. Transmission of Mycobacterium tuberculosis among high school students in Korea. Int J Tuberc Lung Dis. 2001; 5:824–30.
5. Kan B, Berggren I, Ghebremichael S, Bennet R, Bruchfeld J, Chryssanthou E, et al. Extensive transmission of an isoniazid-resistant strain of Mycobacterium tuberculosis in Sweden. Int J Tuberc Lung Dis. 2008; 12:199–204.
6. Tostmann A, Kik SV, Kalisvaart NA, Sebek MM, Verver S, Boeree MJ, et al. Tuberculosis transmission by patients with smear-negative pulmonary tuberculosis in a large cohort in the Netherlands. Clin Infect Dis. 2008; 47:1135–42.

7. van Embden JD, Cave MD, Crawford JT, Dale JW, Eisenach KD, Gicquel B, et al. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993; 31:406–9.

8. Gómez Marín JE, Rigouts L, Villegas Londoño LE, Portaels F. Analysis of restriction fragment length polymorphism (RFLP) in epidemiology of tuberculosis. Bol Oficina Sanit Panam. 1995; 119:1–10.
9. van Soolingen D, Hermans PW, de Haas PE, Soll DR, van Embden JD. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J Clin Microbiol. 1991; 29:2578–86.

10. Glynn JR, Whiteley J, Bifani PJ, Kremer K, van Soolingen D. Worldwide occurrence of Beijing/W strains of Mycobacterium tuberculosis: a systematic review. Emerg Infect Dis. 2002; 8:843–9.
11. European Concerted Action on New Generation Genetic Markers and Techniques for the Epidemiology and Control of Tuberculosis. Beijing/W genotype Mycobacterium tuberculosis and drug resistance. Emerg Infect Dis. 2006; 12:736–43.
12. Rhee JT, Tanaka MM, Behr MA, Agasino CB, Paz EA, Hopewell PC, et al. Use of multiple markers in population-based molecular epidemiologic studies of tuberculosis. Int J Tuberc Lung Dis. 2000; 4:1111–9.
13. Asgharzadeh M, Khakpour M, Salehi TZ, Kafil HS. Use of mycobacterial interspersed repetitive unit-variable-number tandem repeat typing to study Mycobacterium tuberculosis isolates from East Azarbaijan province of Iran. Pak J Biol Sci. 2007; 10:3769–77.
14. Lee AS, Tang LL, Lim IH, Bellamy R, Wong SY. Discrimination of single-copy IS6110 DNA fingerprints of Mycobacterium tuberculosis isolates by high-resolution minisatellite-based typing. J Clin Microbiol. 2002; 40:657–9.
15. Mazars E, Lesjean S, Banuls AL, Gilbert M, Vincent V, Gicquel B, et al. High-resolution minisatellite-based typing as a portable approach to global analysis of Mycobacterium tuberculosis molecular epidemiology. Proc Natl Acad Sci USA. 2001; 98:1901–6.

16. Radhakrishnan I, K MY, Kumar RA, Mundayoor S. Implications of low frequency of IS6110 in fingerprinting field isolates of Mycobacterium tuberculosis from Kerala, India. J Clin Microbiol. 2001; 39:1683.
17. Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, et al. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997; 35:907–14.

18. Gori A, Bandera A, Marchetti G, Degli Esposti A, Catozzi L, Nardi GP, et al. Spoligotyping and Mycobacterium tuberculosis. Emerg Infect Dis. 2005; 11:1242–8.
19. Gori A, Esposti AD, Bandera A, Mezzetti M, Sola C, Marchetti G, et al. Comparison between spoligotyping and IS6110 restriction fragment length polymorphisms in molecular genotyping analysis of Mycobacterium tuberculosis strains. Mol Cell Probes. 2005; 19:236–44.

20. Kwara A, Schiro R, Cowan LS, Hyslop NE, Wiser MF, Roahen Harrison S, et al. Evaluation of the epidemiologic utility of secondary typing methods for differentiation of Mycobacterium tuberculosis isolates. J Clin Microbiol. 2003; 41:2683–5.
21. Filliol I, Ferdinand S, Sola C, Thonnon J, Rastogi N. Spoligotyping and IS6110-RFLP typing of Mycobacterium tuberculosis from French Guiana: a comparison of results with international databases underlines interregional transmission from neighboring countries. Res Microbiol. 2002; 153:81–8.

22. Filliol I, Driscoll JR, Van Soolingen D, Kreiswirth BN, Kremer K, Valétudie G, et al. Global distribution of Mycobacterium tuberculosis spoligotypes. Emerg Infect Dis. 2002; 8:1347–9.

23. Diaz R, Kremer K, de Haas PE, Gomez RI, Marrero A, Valdivia JA, et al. Molecular epidemiology of tuberculosis in Cuba outside of Havana, July 1994–June 1995: utility of spoligotyping versus IS6110 restriction fragment length polymorphism. Int J Tuberc Lung Dis. 1998; 2:743–50.
24. Doroudchi M, Kremer K, Basiri EA, Kadivar MR, Van Soolingen D, Ghaderi AA. IS6110-RFLP and spoligotyping of Mycobacterium tuberculosis isolates in Iran. Scand J Infect Dis. 2000; 32:663–8.
25. Song EJ, Jeong HJ, Lee SM, Kim CM, Song ES, Park YK, et al. A DNA chip-based spoligotyping method for the strain identification of Mycobacterium tuberculosis isolates. J Microbiol Methods. 2007; 68:430–3.

26. Supply P, Allix C, Lesjean S, Cardoso-Oelemann M, Rüsch-Gerdes S, Willery E, et al. Proposal for standardization of optimized mycobacterial interspersed repetitive unit-variable-number tandem repeat typing of Mycobacterium tuberculosis. J Clin Microbiol. 2006; 44:4498–510.
27. Oelemann MC, Diel R, Vatin V, Haas W, Rüsch-Gerdes S, Locht C, et al. Assessment of an optimized mycobacterial interspersed repetitive- unit-variable-number tandem-repeat typing system combined with spoligotyping for population-based molecular epidemiology studies of tuberculosis. J Clin Microbiol. 2007; 45:691–7.

28. Bouakaze C, Keyser C, de Martino SJ, Sougakoff W, Veziris N, Dabernat H, et al. Identification and genotyping of Mycobacterium tuberculosis complex species by use of a SNaPshot Minise-quencing-based assay. J Clin Microbiol. 2010; 48:1758–66.
29. Mahasirimongkol S, Yanai H, Nishida N, Ridruechai C, Matsushita I, Ohashi J, et al. Genome-wide SNP-based linkage analysis of tuberculosis in Thais. Genes Immun. 2009; 10:77–83.

30. van Soolingen D, Qian L, de Haas PE, Douglas JT, Traore H, Portaels F, et al. Predominance of a single genotype of Mycobacterium tuberculosis in countries of east Asia. J Clin Microbiol. 1995; 33:3234–8.

31. Park YK, Bai GH, Kim SJ. Restriction fragment length polymorphism analysis of Mycobacterium tuberculosis isolated from countries in the western pacific region. J Clin Microbiol. 2000; 38:191–7.
32. Kang HY, Wada T, Iwamoto T, Maeda S, Murase Y, Kato S, et al. Phylogeographical particularity of the Mycobacterium tuberculosis Beijing family in South Korea based on international comparison with surrounding countries. J Med Microbiol. 2010; 59:1191–7.

33. Choi GE, Jang MH, Song EJ, Jeong SH, Kim JS, Lee WG, et al. IS6110-restriction fragment length polymorphism and spoligotyping analysis of Mycobacterium tuberculosis clinical isolates for investigating epidemiologic distribution in Korea. J Korean Med Sci. 2010; 25:1716–21.
34. Shamputa IC, Lee J, Allix-Béguec C, Cho EJ, Lee JI, Rajan V, et al. Genetic diversity of Mycobacterium tuberculosis isolates from a tertiary care tuberculosis hospital in South Korea. J Clin Microbiol. 2010; 48:387–94.
35. Jeon CH, Lee SC, Sohn JH, Ahn WS. Restriction fragment length polymorphism analysis of Mycobacterium tuberculosis isolated from Taegu. Korean J Clin Pathol. 1999; 19:581–6.
36. Park YK, Kang HY, Lim JG, Ha JS, Cho JO, Lee KC, et al. Analysis of DNA fingerprints of Mycobacterium tuberculosis isolates from patients registered at health center in Gyeonggi Province in 2004. Tuberc Respir Dis. 2006; 60:290–6.

37. Bifani PJ, Mathema B, Kurepina NE, Kreiswirth BN. Global dissemination of the Mycobacterium tuberculosis W-Beijing family strains. Trends Microbiol. 2002; 10:45–52.

38. Qian L, Abe C, Lin TP, Yu MC, Cho SN, Wang S, et al. rpoB genotypes of Mycobacterium tuberculosis Beijing family isolates from East Asian countries. J Clin Microbiol. 2002; 40:1091–4.
39. Park YK, Shin S, Ryu S, Cho SN, Koh WJ, Kwon OJ, et al. Comparison of drug resistance genotypes between Beijing and non-Beijing family strains of Mycobacterium tuberculosis in Korea. J Microbiol Methods. 2005; 63:165–72.

40. Chang CL, Kim HH, Son HC, Park SS, Lee MK, Park SK, et al. False-positive growth of Mycobacterium tuberculosis attributable to laboratory contamination confirmed by restriction fragment length polymorphism analysis. Int J Tuberc Lung Dis. 2001; 5:861–7.
41. Yun KW, Song EJ, Choi GE, Hwang IK, Lee EY, Chang CL. Strain typing of Mycobacterium tuberculosis isolates from Korea by mycobacterial interspersed repetitive units-variable number of tandem repeats. Korean J Lab Med. 2009; 29:314–9.

42. Hanekom M, van der Spuy GD, Gey van Pittius NC, McEvoy CR, Hoek KG, Ndabambi SL, et al. Discordance between mycobacterial interspersed repetitive-unit-variable-number tandem-repeat typing and IS6110 restriction fragment length polymorphism genotyping for analysis of Mycobacterium tuberculosis Beijing strains in a setting of high incidence of tuberculosis. J Clin Microbiol. 2008; 46:3338–45.
43. Filliol I, Motiwala AS, Cavatore M, Qi W, Hazbón MH, Bobadilla del Valle M, et al. Global phylogeny of Mycobacterium tuberculosis based on single nucleotide polymorphism (SNP) analysis: insights into tuberculosis evolution, phylogenetic accuracy of other DNA fingerprinting systems, and recommendations for a minimal standard SNP set. J Bacteriol. 2006; 188:759–72.
44. Choi GE, Jang MH, Cho HJ, Lee SM, Yi J, Lee EY, et al. Application of single-nucleotide polymorphism and mycobacterial interspersed repetitive units-variable number of tandem repeats analyses to clinical Mycobacterium tuberculosis isolates from Korea. Korean J Lab Med. 2011; 31:37–43.
Go to : 
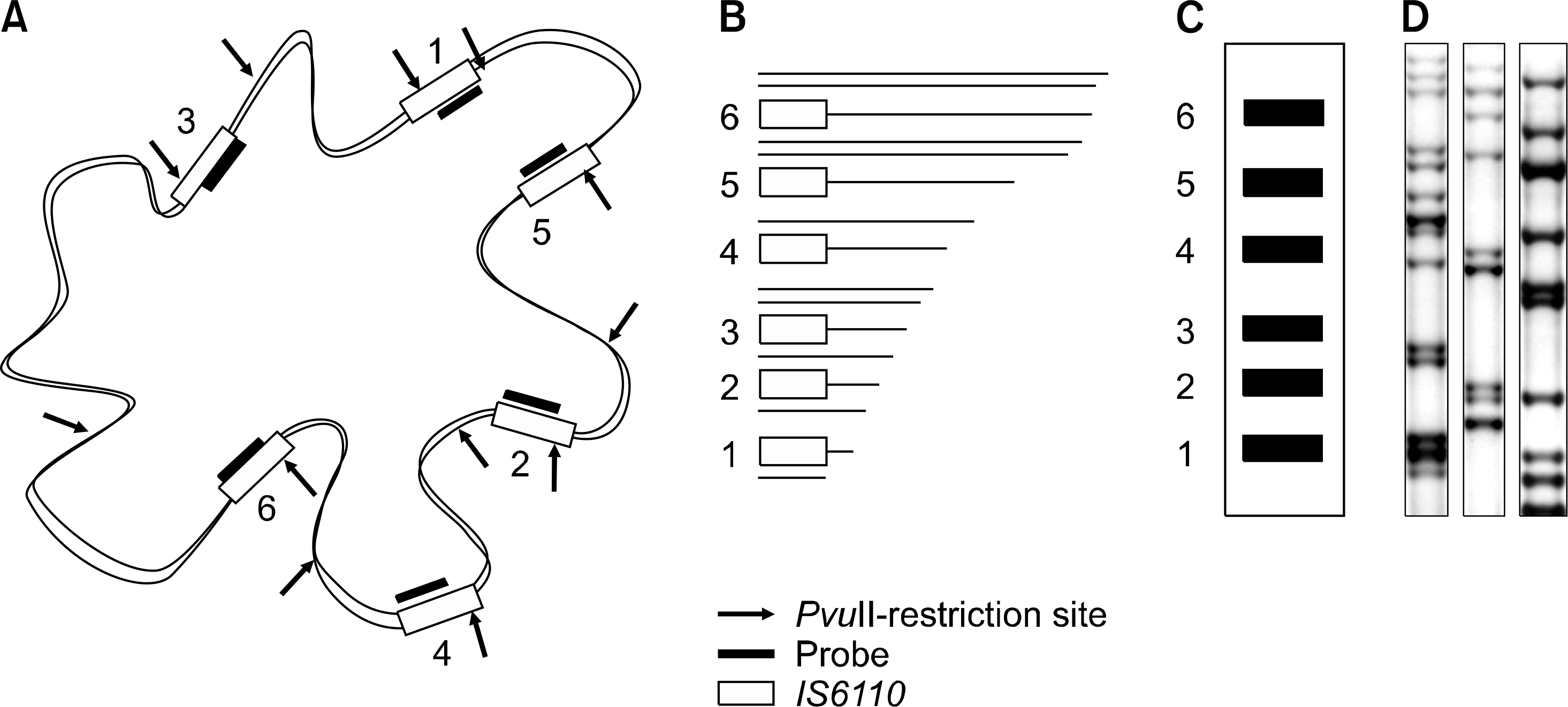 | Fig. 1.The principles and procedures of IS6110-RFLP. (A) IS6110-RFLP for epidemiological investigation used the characteristics of polymorphism and copy number of IS6110. Extracted genomic DNA of M. tuberculosis was digested with a frequently cutting restriction enzyme, PvuII. IS6110 has only one restriction site for PvuII. Chromosomal regions other than IS6110 have randomly dispersed multiple restriction sites for PvuII. Therefore, lengths of fragments containing one end of IS6110 are variable; (B) DNA fragments under well-defined conditions were separated on agarose gel, and transferred to a nylon membrane; (C) hybridization with a fragment of the IS6110 sequence developed only IS6110-containing bands; (D) an image of real IS6110-RFLP patterns. |
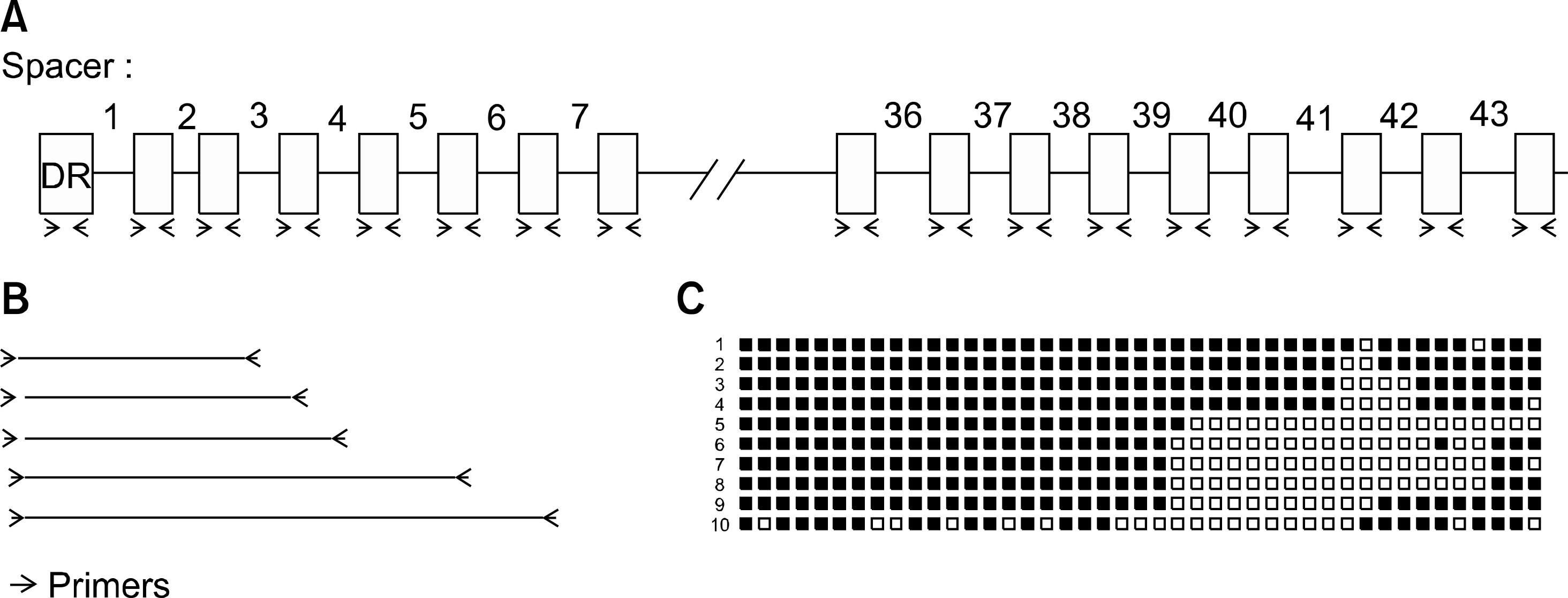 | Fig. 2.The principles and procedures of DR spoligotyping. (A) Spoligotyping is based on the polymorphism in the direct repeat locus of the mycobacterial chromosome. The well-conserved 36-bp direct repeats are interspersed with unique spacer sequences varying from 35 to 41 bp in size. Deletion of some spacer regions between DRs is the main cause of strain diversity. Because the DRs have the same sequence, PCR generates fragments containing spacer sequences that are present in a specific strain; (B) Each PCR fragment contained at least one spacer sequence, although it may have many spacer sequences; (C) In a membrane, every probes targeting each spacer sequencer are spotted. PCR fragments are hybridized to the spots. The presence or absence of each spot generated unique spoligotyping pattern. |
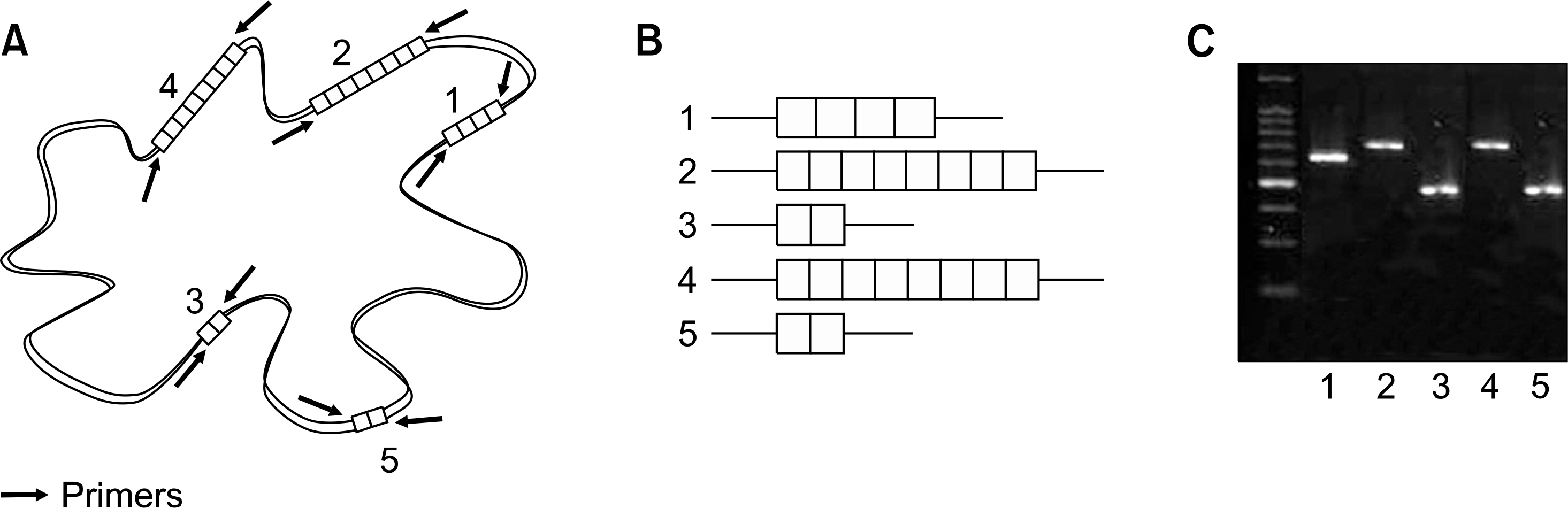 | Fig. 3.The principles and procedures of MIRU-VNTR. (A) MIRU-VNTRs scattered throughout the whole bacterial genome contain variable copy numbers of repeat sequences. Each isolate is typed according to the number of copies of repeated units; (B) Sequences of variable number tandem repeats are amplified by PCR. The sizes of the amplified produces are dependent on the repeat copy numbers; (C) their sizes are determined by gel electrophoresis. |
Table 1.
Characteristics of molecular strain typing methods fre quently used for M. tuberculosis
Table 2.
Frequency of Beijing family of M. tuberculosis in Korea




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download