Abstract
Background
Asymptomatic vancomycin-resistant enterococci (VRE) colonization precedes infection. VRE-colonized patients serve as silent reservoirs of enterococci that go on to colonize other patients. Rapidly identifying colonized patients is crucial to prevent the spread of VRE. The culture-based method of VRE screening is time-consuming. We evaluated the diagnostic performance of a recently developed multiplex realtime PCR for the detection of VRE.
Methods
We obtained 105 rectal swabs from patients who were being monitored for carriage of VRE. After 24 hour incubation of swabs in enterococcosel broth (EB) supplemented with 6 μg/mL vancomycin, multiplex realtime PCR was performed using the Anyplex TM VanR Real-time Detection (VanR) kit (Seegene, Inc., Seoul, Korea). The results of multiplex realtime PCR were compared to those of culture. We evaluated the specificity and detection limits of multiplex realtime PCR using VanR for VRE.
Results
A total of 96/105 (91.4%) samples were VRE positive according to multiplex realtime PCR with EB while 85/105 (80.9%) samples were positive in culture. Eleven discordant results (10.4%) (multiplex realtime PCR positive, culture negative) were noted. All non-enterococcal bacteria and vancomycin-susceptible enterococci were negative. The DNA detection limits of VanR were 0.035 pg per reaction (3 μL) for Enterococcus faecium and 0.35 pg for Enterococcus faecalis.
Go to : 
REFERENCES
1. Roghmann MC, Qaiyumi S, Schwalbe R, Morris JG Jr. Natural history of colonization with vancomycin-resistant Enterococcus faecium. Infect Control Hosp Epidemiol. 1997; 18:679–80.

2. Handwerger S and Skoble J. Identification of chromosomal mobile element conferring high-level vancomycin resistance in Enterococcus faecium. Antimicrob Agents Chemother. 1995; 39:2446–53.

3. Simonsen GS, Haaheim H, Dahl KH, Kruse H, L⊘vseth A, Olsvik O, et al. Transmission of VanA-type vancomycin-resistant enterococci and vanA resistance elements between chicken and humans at avoparcin-exposed farms. Microb Drug Resist. 1998; 4:313–8.
4. Lee WG, Jernigan JA, Rasheed JK, Anderson GJ, Tenover FC. Possible horizontal transfer of the vanB2 gene among genetically diverse strains of vancomycin-resistant Enterococcus faecium in a Korean hospital. J Clin Microbiol. 2001; 39:1165–8.
5. Lee WG. Resistance mechanism and epidemiology of vancomycin-resistanct enterococci. Korean J Clin Microbiol. 2008; 11:71–7.
6. Hospital Infection Control Practices Advisory Committee (HICP-AC). Recommendations for preventing the spread of vancomycin resistance. Infect Control Hosp Epidemiol. 1995; 16:105–13.
7. Dutka-Malen S, Evers S, Courvalin P. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J Clin Microbiol. 1995; 33:1434.

8. Fluit AC, Visser MR, Schmitz FJ. Molecular detection of antimicrobial resistance. Clin Microbiol Rev. 2001; 14:836–71.

9. Sahm DF, Free L, Smith C, Eveland M, Mundy LM. Rapid characterization schemes for surveillance isolates of vancomycin-resistant enterococci. J Clin Microbiol. 1997; 35:2026–30.

10. D'Agata EM, Gautam S, Green WK, Tang YW. High rate of false-negative results of the rectal swab culture method in detection of gastrointestinal colonization with vancomycin-resistant enterococci. Clin Infect Dis. 2002; 34:167–72.
11. Palladino S, Kay ID, Flexman JP, Boehm I, Costa AM, Lambert EJ, et al. Rapid detection of vanA and vanB genes directly from clinical specimens and enrichment broths by realtime multiplex PCR assay. J Clin Microbiol. 2003; 41:2483–6.
12. Donskey CJ, Hoyen CK, Das SM, Helfand MS, Hecker MT. Recurrence of vancomycin-resistant enterococcus stool colonization during antibiotic therapy. Infect Control Hosp Epidemiol. 2002; 23:436–40.
13. Lee WG, Park IJ, Jin HY, Park MH. Relapse and reacquisition of rectal colonization by vancomycin-resistant Enterococcus faecium after decolonization. Epidemiol Infect. 2010; 138:1449–53.
14. Toye B, Shymanski J, Bobrowska M, Woods W, Ramotar K. Clinical and epidemiological significance of enterococci intrinsically resistant to vancomycin (possessing the vanC genotype). J Clin Microbiol. 1997; 35:3166–70.

15. Satake S, Clark N, Rimland D, Nolte FS, Tenover FC. Detection of vancomycin-resistant enterococci in fecal samples by PCR. J Clin Microbiol. 1997; 35:2325–30.

16. Drews SJ, Johnson G, Gharabaghi F, Roscoe M, Matlow A, Tellier R, et al. A 24-hour screening protocol for identification of vancomycin-resistant Enterococcus faecium. J Clin Microbiol. 2006; 44:1578–80.
17. Morata P, Queipo-Ortuño MI, de Dios Colmenero J. Strategy for optimizing DNA amplification in a peripheral blood PCR assay used for diagnosis of human brucellosis. J Clin Microbiol. 1998; 36:2443–6.

18. Kim DH, Lee JH, Ha JS, Ryoo NH, Jeon DS, Kim JR. Evaluation of the usefulness of selective chromogenic agar medium (chromID VRE) and multiplex PCR method for the detection of vancomycin-resistant enterococci. Korean J Lab Med. 2010; 30:631–6.

19. Roger M, Faucher MC, Forest P, St-Antoine P, Coutlée F. Evaluation of a vanA-specific PCR assay for detection of vancomycin-resistant Enterococcus faecium during a hospital outbreak. J Clin Microbiol. 1999; 37:3348–9.
20. Kim S, Sung H, Jeon HS, Park SJ, Park SH, Kim MN. Evaluation of a rapid enrichment-PCR method for the detection of vanA vancomycin-resistant enterococci in fecal specimenss. Korean J Clin Microbiol. 2007; 10:44–8.
Go to : 
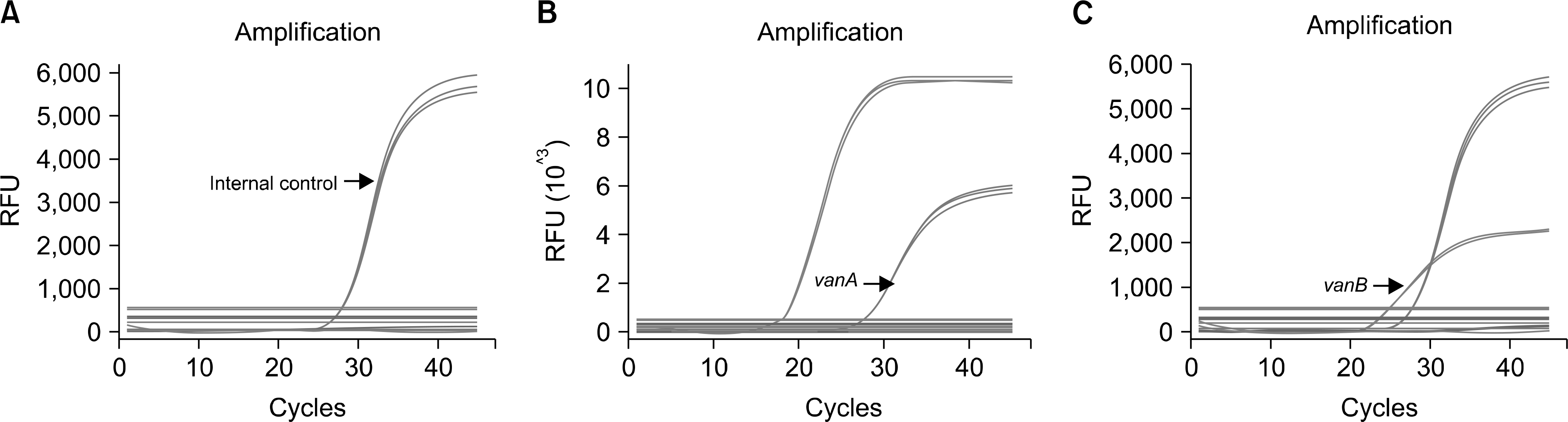 | Fig. 1.Results of multiplex realtime PCR for the detection of VRE in triplicate. (A) E. faecalis (ATCC 29212). (B) E. faecium (BM 4147, vanA). (C) E. faecalis (ATCC 51299, vanB). |
Table 1.
Comparative results of multiplex realtime PCR using AnyplexTM VanR Real-time Detection kit and culture method for the detection of VRE
| VRE detection | Multiplex realtime PCR | Total No. (%) of specimens | |
|---|---|---|---|
| Positive | Negative | ||
| Culture | |||
| Positive | 85 (80.9%) | 0 (0%) | 85 (80.9%) |
| Negative | 11 (10.4%)∗ | 9 (8.5%) | 20 (19.1%) |
| Total No. (%) | 96 (91.4%) | 9 (8.5%) | 105 (100%) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download