Abstract
Background
Chlamydophila pneumoniae is one of the major respiratory infectious pathogens and can be accurately diagnosed by cell culturing. The author performed this study to compare the usefulness of the collagen-coated polyethylene terephthalate (PET) disc culture method and that of the shell vial method.
Methods
Twenty-nine sputums and 17 blood specimens collected from 46 patients for C. pneumoniae culture were inoculated into HeLa-229 cell monolayers cultured in shell vials and polyester plates. After incubation, they were stained using the indirect immunofluorescent method with genus-specific FITC-conjugated anti-chlamydia antibody. When both results were inconsistent, microimmunofluorescence results were used.
Results
HeLa-229 cells successfully formed monolayers in shell vials and collagen-coated PET plates in all cases. Positive inclusion bodies in HeLa-229 cells of shell vials and PET plates for C. pneumoniae culture were similarly stained with the indirect immunofluorescent method. Both methods showed consistent results with 20 positive and 22 negative cases. The total agreement between the PET plate and shell vial was excellent (91.3%, k=0.826).
Conclusion
The collagen-coated PET disc culture method showed highly consistent results with that of the shell vial method, and no technical differences were experienced between the two methods. Therefore, the author concluded that the shell vial method could be replaced by the PET plate method for detection of C. pneumoniae.
REFERENCES
1. Hammerschlag MR. The intracellular life of chlamydiae. Semin Pediatr Infect Dis. 2002; 13:239–48.

2. Everett KD, Bush RM, Andersen AA. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int J Syst Bacteriol. 1999; 49:415–40.

3. Chi EY, Kuo CC, Grayston JT. Unique ultrastructure in the elementary body of Chlamydia sp. strain TWAR. J Bacteriol. 1987; 169:3757–63.

4. Kuo CC, Jackson LA, Campbell LA, Grayston JT. Chlamydia pneumoniae (TWAR). Clin Microbiol Rev. 1995; 8:451–61.

6. Wong YK, Dawkins KD, Ward ME. Circulating Chlamydia pneumoniae DNA as a predictor of coronary artery disease. J Am Coll Cardiol. 1999; 34:1435–9.
7. Layh-Schmitt G, Bendl C, Hildt U, Dong-Si T, Jüttler E, Schnitzler P, et al. Evidence for infection with Chlamydia pneumoniae in a subgroup of patients with multiple sclerosis. Ann Neurol. 2000; 47:652–5.
8. Balin BJ, Gérard HC, Arking EJ, Appelt DM, Branigan PJ, Abrams JT, et al. Identification and localization of Chlamydia pneumoniae in the Alzheimer's brain. Med Microbiol Immunol. 1998; 187:23–42.

9. Cascina A, Marone Bianco A, Mangiarotti P, Montecucco CM, Meloni F. Cutaneous vasculitis and reactive arthritis following respiratory infection due to Chlamydia pneumoniae: report of a case. Clin Exp Rheumatol. 2002; 20:845–7.
10. Hahn DL, Peeling RW, Dillon E, McDonald R, Saikku P. Serologic markers for Chlamydia pneumoniae in asthma. Ann Allergy Asthma Immunol. 2000; 84:227–33.

11. Dowell SF, Peeling RW, Boman J, Carlone GM, Fields BS, Guarner J, et al. Standardizing Chlamydia pneumoniae assays: recommendations from the Centers for Disease Control and Prevention (USA) and the Laboratory Centre for Disease Control (Canada). Clin Infect Dis. 2001; 33:492–503.
12. Kumar S and Hammerschlag MR. Acute respiratory infection due to Chlamydia pneumoniae: current status of diagnostic methods. Clin Infect Dis. 2007; 44:568–76.
13. Kim KS, Choi TY, Sohn HE. A study for culture condition of Chlamydia pneumoniae. Korean J Clin Pathol. 1997; 17:137–45.
14. Frobes BA, Sahm DF, et al. eds. Bailey & Scott's Diagnostic Microbiology. 12th ed.St. Louis: Mosby;2006. p. 756–59.
15. Kuo CC and Grayston JT. Factors affecting viability and growth in HeLa 229 cells of Chlamydia sp. strain TWAR. J Clin Microbiol. 1988; 26:812–5.

16. Maass M, Essig A, Marre R, Henkel W. Growth in serum-free medium improves isolation of Chlamydia pneumoniae. J Clin Microbiol. 1993; 31:3050–2.

17. Pruckler JM, Masse N, Stevens VA, Gang L, Yang Y, Zell ER, et al. Optimizing culture of Chlamydia pneumoniae by using multiple centrifugations. J Clin Microbiol. 1999; 37:3399–401.
18. Tjhie JH, Roosendaal R, MacLaren DM, Vandenbroucke-Grauls CM. Improvement of growth of Chlamydia pneumoniae on HEp-2 cells by pretreatment with polyethylene glycol in combination with additional centrifugation and extension of culture time. J Clin Microbiol. 1997; 35:1883–4.

19. Kazuyama Y, Lee SM, Amamiya K, Taguchi F. A novel method for isolation of Chlamydia pneumoniae by treatment with trypsin or EDTA. J Clin Microbiol. 1997; 35:1624–6.

20. Roblin PM, Dumornay W, Hammerschlag MR. Use of HEp-2 cells for improved isolation and passage of Chlamydia pneumoniae. J Clin Microbiol. 1992; 30:1968–71.

21. Chirgwin K, Roblin PM, Gelling M, Hammerschlag MR, Schachter J. Infection with Chlamydia pneumoniae in Brooklyn. J Infect Dis. 1991; 163:757–61.

22. Boman J, Allard A, Persson K, Lundborg M, Juto P, Wadell G. Rapid diagnosis of respiratory Chlamydia pneumoniae infection by nested touchdown polymerase chain reaction compared with culture and antigen detection by EIA. J Infect Dis. 1997; 175:1523–6.
23. Verkooyen RP, Willemse D, Hiep-van Casteren SC, Joulandan SA, Snijder RJ, van den Bosch JM, et al. Evaluation of PCR, culture, and serology for diagnosis of Chlamydia pneumoniae respiratory infections. J Clin Microbiol. 1998; 36:2301–7.
24. Apfalter P, Boman J, Nehr M, Hienerth H, Makristathis A, Pauer J, et al. Application of blood-based polymerase chain reaction for detection of Chlamydia pneumoniae in acute respiratory tract infections. Eur J Clin Microbiol Infect Dis. 2001; 20:584–6.

Fig. 1.
(A) Shell vial and round cover glass (12 mm). The cover-glass is located onto the bottom of the shell vial. (B) Collagen-coated polyethylene terephthalate (PET) plate (4-well) and PET disc (15 mm). The PET discs are located onto the bottoms of the wells.
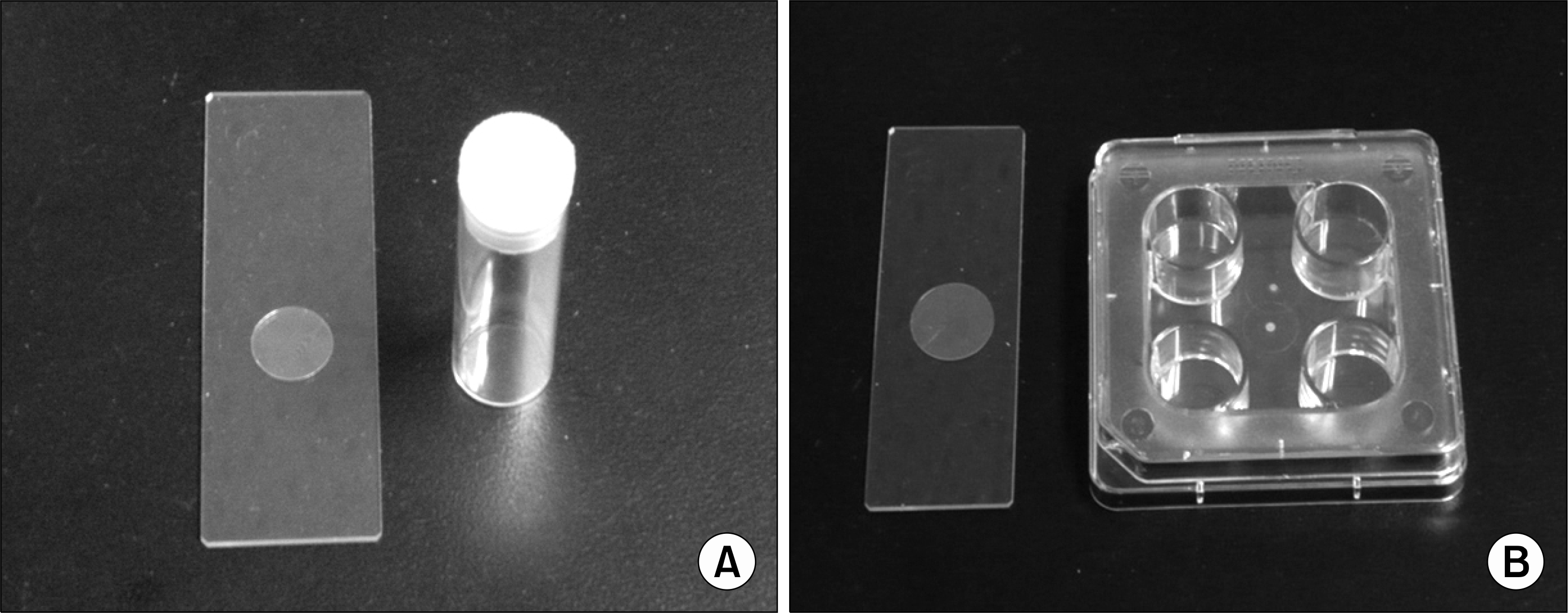
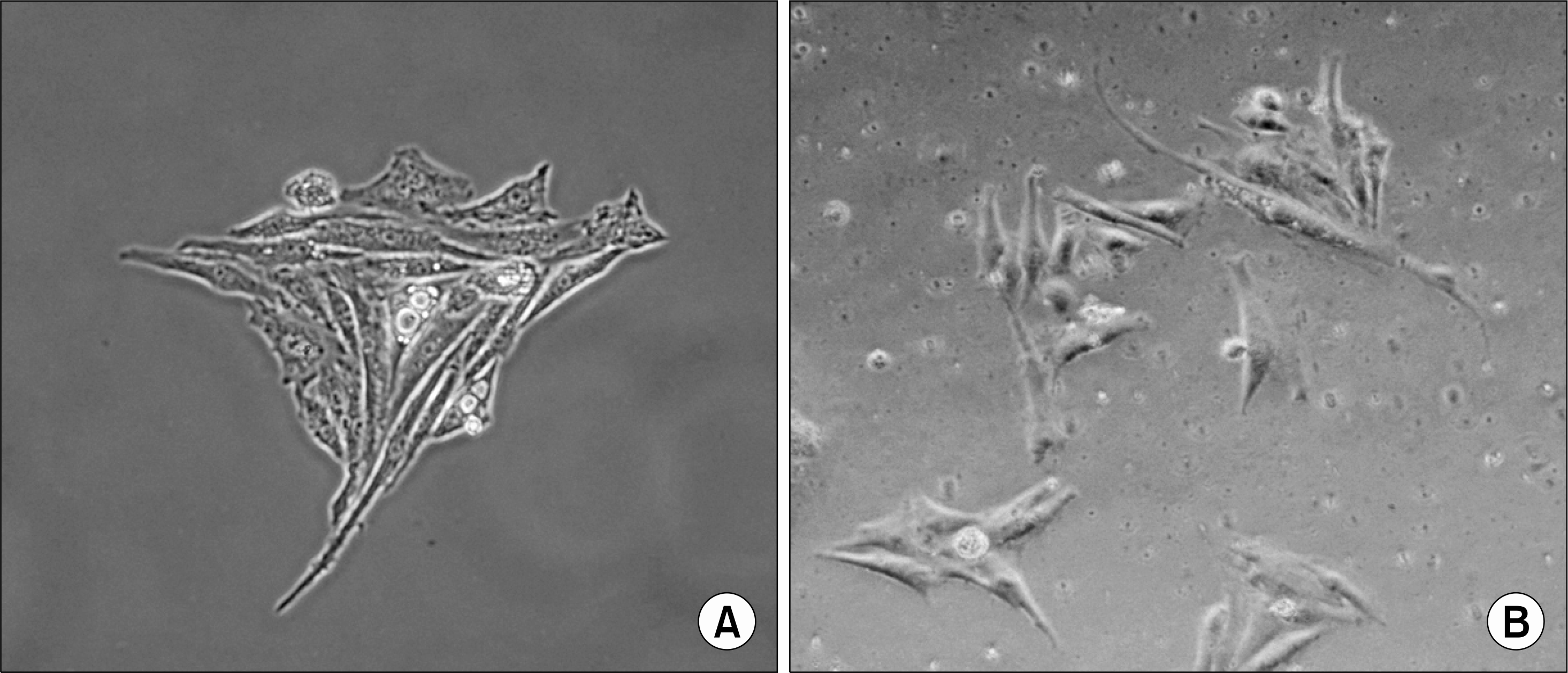
Fig. 2.
A monolayer of HeLa-229 cells with the unoccupied surface and cell boundary and the condensed nuclear chromatin using inverted phase-contrast microscopy (×400). The HeLa-229 cells were cultivated at 37°C with 5% CO2 in shell vial (A) and collagen-coated polyethylene terephthalate (PET) plate (B).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download