Abstract
Background
Recently hepatitis A virus (HAV) infection has propagated among adults in Korea due to the epidemiologic shift in the age-specific HAV seroprevalence. There are apparently increase in symptomatic patients with severe diseases. This study aimed to investigate clinical and molecular characteristics related to acute kidney injury (AKI) occurrence in HAV infection.
Methods
A case-control study was conducted in a university hospital between February 2009 and July 2009. Clinical findings of non-fulminant HAV infection complicated with AKI (N=5) were compared to those without AKI (N=60). The complete sequence of the VP1 region (900 bp) was amplified from stool specimens by the RT-PCR to determine HAV genotypes and genetic variations between the two groups.
Results
Among 65 patients with non-fulminant HAV infections, 5 patients (7.7%) developed AKI. In multivariate analyses, higher level of C-reactive protein was independently associated with AKI occurrence in non-fulminant HAV infections [odds ratios=1.094, 95% confidence interval=1.011−1.183]. HAV RNA was detected in 57 (86.4%) patients: 53 strains (93.0%) showed genotype IIIA and 4 strains presented genotype IA. All HAV isolates from the AKI patients belonged to the genotype IIIA and shared the identical sequences with those from the non-AKI patients.
Go to : 
REFERENCES
1. Song MH, Lim YS, Song TJ, Choi JM, Kim JI, Jun JB, et al. The etiology of acute viral hepatitis for the last 3 years. Korean J Med. 2005; 68:256–60.
2. Yoon YK, Chun BC, Lee HK, Seo YS, Shin JH, Hong YS, et al. Epidemiological and genetic analysis of a sustained community-wide outbreak of hepatitis A in the Republic of Korea, 2008: a hospital-based case-control study. J Clin Virol. 2009; 46:184–8.

3. Park CH, Cho YK, Park JH, Jun JS, Park ES, Seo JH, et al. Changes in the age-specific prevalence of hepatitis A virus antibodies: a 10-year cohort study in Jinju, South Korea. Clin Infect Dis. 2006; 42:1148–50.

4. Kang CI, Choi CM, Park TS, Lee DJ, Oh MD, Choe KW. Incidence and seroprevalence of hepatitis A virus infections among young Korean soldiers. J Korean Med Sci. 2007; 22:546–8.

5. Willner IR, Uhl MD, Howard SC, Williams EQ, Riely CA, Waters B. Serious hepatitis A: an analysis of patients hospitalized during an urban epidemic in the United States. Ann Intern Med. 1998; 128:111–4.

6. Kim KH, Lee TH, Yang JK, Kim SM, Im EH, Huh KC, et al. Two cases of acute renal failure associated with nonfulminant acute hepatitis A. Korean J Gastroenterol. 2007; 50:116–20.
7. Kim HW, Yu MH, Lee JH, Chang JW, Yang WS, Kim SB, et al. Experiences with acute kidney injury complicating non-fulminant hepatitis A. Nephrology (Carlton). 2008; 13:451–8.

8. Jung YJ, Kim W, Jeong JB, Kim BG, Lee KL, Oh KH, et al. Clinical features of acute renal failure associated with hepatitis A virus infection. J Viral Hepat. 2009. [Epub ahead of print].

9. Kim SH, Yoon HE, Kim YK, Kim JY, Choi BS, Choi YJ, et al. Acute hepatitis A-associated acute renal failure in adults. Nephron Clin Pract. 2008; 109:127–32.

10. Nainan OV, Xia G, Vaughan G, Margolis HS. Diagnosis of hepatitis a virus infection: a molecular approach. Clin Microbiol Rev. 2006; 19:63–79.

11. Brenner. The Kidney. 5th ed.Philadelphia: W.B. Saunders Company;2006. p. 1200–52.
12. Wilkinson SP, Davies MH, Portmann B, Williams R. Renal failure in otherwise uncomplicated acute viral hepatitis. Br Med J. 1978; 2:338–41.

14. Green J, Beyar R, Bomzon L, Finberg JP, Better OS. Jaundice, the circulation and the kidney. Nephron. 1984; 37:145–52.

15. Green J, Better OS. Systemic hypotension and renal failure in obstructive jaundice-mechanistic and therapeutic aspects. J Am Soc Nephrol. 1995; 5:1853–71.

16. Fan PC, Chen YC, Tian YC, Chang CH, Fang JT, Yang CW. Acute renal failure associated with acute non-fulminant hepatitis A: a case report and review of literature. Ren Fail. 2009; 31:756–64.

17. Lin CC, Chang CH, Lee SH, Chiang SS, Yang AH. Acute renal failure in non-fulminant hepatitis A. Nephrol Dial Transplant. 1996; 11:2061–6.

18. Morita M, Kitajima K, Yoshizawa H, Itoh Y, Iwakiri S, Shibata C, et al. Glomerulonephritis associated with arteritis in marmosets infected with hepatitis A virus. Br J Exp Pathol. 1981; 62:103–13.
19. Sasaki K, Fujita I, Hamasaki Y, Miyazaki S. Differentiating between bacterial and viral infection by measuring both C-reactive protein and 2'-5'-oligoadenylate synthetase as inflammatory markers. J Infect Chemother. 2002; 8:76–80.

20. Atono Y, Sata M, Tanikawa K. Kinetics of C-reactive protein in acute viral hepatitis. Gastroenterol Jpn. 1989; 24:655–62.

21. Pecoits-Filho R, Heimbürger O, Bárány P, Suliman M, Fehrman-Ekholm I, Lindholm B, et al. Associations between circulating inflammatory markers and residual renal function in CRF patients. Am J Kidney Dis. 2003; 41:1212–8.

22. Ortega O, Rodriguez I, Gallar P, Carreño A, Ortiz M, Espejo B, et al. Significance of high C-reactive protein levels in pre-dialysis patients. Nephrol Dial Transplant. 2002; 17:1105–9.

23. Harrison NA, Masterton RG, Bateman JM, Rainford DJ. C-reactive protein in acute renal failure. Nephrol Dial Transplant. 1989; 4:864–9.

24. Robertson BH, Jansen RW, Khanna B, Totsuka A, Nainan OV, Siegl G, et al. Genetic relatedness of hepatitis A virus strains recovered from different geographical regions. J Gen Virol. 1992; 73:1365–77.

25. Comeron JM. A method for estimating the numbers of synonymous and nonsynonymous substitutions per site. J Mol Evol. 1995; 41:1152–9.

26. Byun KS, Kim JH, Song KJ, Baek LJ, Song JW, Park SH, et al. Molecular epidemiology of hepatitis A virus in Korea. J Gastroenterol Hepatol. 2001; 16:519–24.
27. Yun H, Kim S, Lee H, Byun KS, Kwon SY, Yim HJ, et al. Genetic analysis of HAV strains isolated from patients with acute hepatitis in Korea, 2005-2006. J Med Virol. 2008; 80:777–84.

28. Fujiwara K, Yokosuka O, Imazeki F, Saisho H, Saotome N, Suzuki K, et al. Analysis of the genotype-determining region of hepatitis A viral RNA in relation to disease severity. Hepatology Res. 2003; 25:124–34.
Go to : 
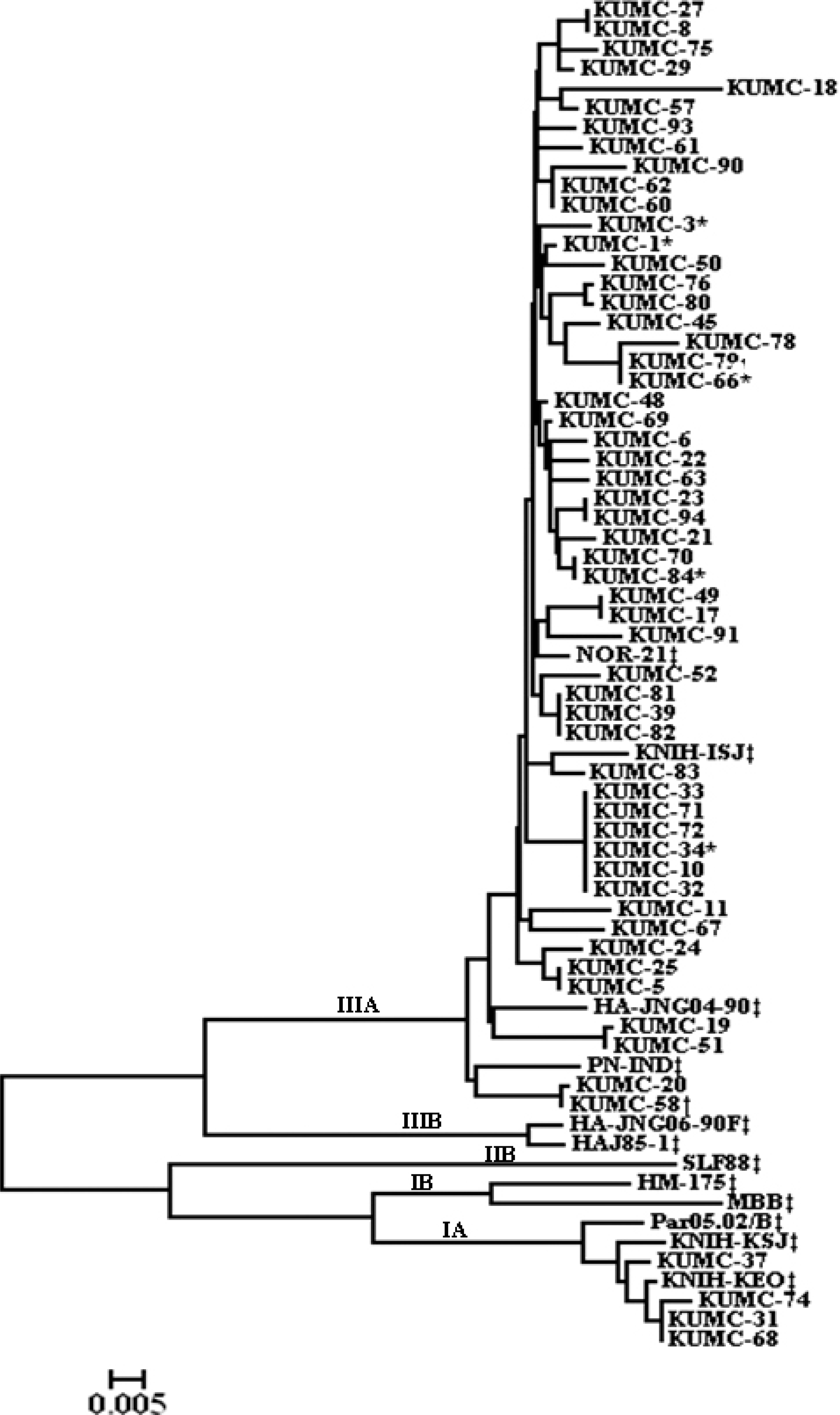 | Fig. 1.The phylogenetic tree constructed for the nucleotide sequences of VP1 region of hepatitis A virus genome. The tree includes 66 strains isolated from this study and the reference strains. ∗The strains isolated from the non-fulminant hepatitis with acute kidney injury. †The strains isolated from the fulminant hepatitis. ‡The reference strains. |
Table 1.
The primer sequences used for amplification of the VP1 region of the hepatitis A virus genome by reverse transcriptase-polymerase chain reaction
| Primers | Position∗ | Nucleotide sequences | The predicted size (bp) |
|---|---|---|---|
| VP1-1N-F | 2184-2203 | 5′ CATGC(T/C)ATGGATGT(C/T)AC(A/C/T)AC 3″ | 1,010 |
| VP1-2-R | 3174-3193 | 5′ GACARYTCTTCYTGAGCATA 3′ | |
| VP1-A-F | 2122-2145 | 5′ CTCATGTTAGAGTTAATGTTTATC 3′ | 1,063 |
| VP1-A-R | 3164-3184 | 5′ TCCTGAGCATATTTGAGTCTT 3′ | |
| VP1-AN-F | 2133-2155 | 5′ GTTAATGTTTATCTTTCAGCAAT 3′ | 987 |
| VP1-AN-R | 3098-3119 | 5′ TTCTATATGACTCTCAAATCTT 3′ |
Table 2.
Comparative analyses of demographic and clinical features between the non-fulminant HAV infections with or without acute kidney injury
| Variables | Cases (N=5) | Controls (N=60) | Univariate P | Multivariate OR∗ (95% CI†) |
|---|---|---|---|---|
| Male, n (%) | 5 (100) | 34 (56.7) | 0.078 | |
| Age, years | 39.0±2.9 | 33.3±0.85 | 0.066 | 1.046 (0.806−1.357) |
| Malignancy | 0 | 1 (1.7) | 1.000 | |
| History of hepatitis B carrier | 0 | 1 (1.7) | 1.000 | |
| Genotype, IIIA | 5 (100) | 47 (92.2) | 1.000 | |
| Symptom/sign | ||||
| Fever | 4 (80.0) | 48 (80.0) | 1.000 | |
| Fatigue | 3 (60.0) | 39 (65.0) | 1.000 | |
| Myalgia | 1 (20.0) | 17 (28.3) | 1.000 | |
| Nausea/vomiting | 4 (80.0) | 47 (78.3) | 1.000 | |
| Abdominal discomfort | 2 (40.0) | 25 (41.7) | 1.000 | |
| Headache | 0 | 10 (16.7) | 1.000 | |
| Jaundice | 3 (60.0) | 6 (10.0) | 0.017 | |
| Diarrhea | 1 (20.0) | 7 (11.7) | 0.493 | |
| Laboratory finding (peak) | ||||
| WBC, ∗109/L | 6.0±2.1 | 4.6±2.1 | 0.168 | |
| Platelets, ∗109/L | 185.2±48.1 | 160.2±69.8 | 0.436 | |
| PT, INR | 1.3±0.5 | 1.3±0.3 | 0.787 | |
| ALT, U/L | 2,965±1,586 | 4,077±2,739 | 0.161 | |
| AST, U/L | 3,833±3,217 | 2,477±1,925 | 0.157 | |
| TB, mg/dL | 15.6±3.6 | 7.4±5.9 | 0.004 | 1.058 (0.810−1.382) |
| Albumin, g/dL | 3.2±0.4 | 3.6±0.4 | 0.035 | 1.413 (0.190−104.317) |
| Cr, mg/dL | 11.14±4.9 | 0.9±0.2 | 0.000 | |
| CRP, mg/dL | 70.3±30.2 | 18.5±17.3 | 0.000 | 1.094 (1.011−1.183) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download