Abstract
Background
Increasing numbers of Acinetobacter spp. resistant to multiple drugs, including carbapenem, has been a serious problem. The aims of this study were to determine carbapenem resistance patterns and mechanisms, as well as to study the molecular epidemiology of Acinetobacter spp.
Methods
Clinical isolates of Acinetobacter spp. were collected from May to November in 2006. Antimicrobial susceptibility testing was performed using CLSI disk diffusion and agar dilution methods. Metallo-β-lactamase-and OXA carbapenemase-producing isolates were detected by PCR. Carbapenem resistance and hydrolytic activities were compared according to OXA type and presence of ISAba1. Pulsed-field gel electrophoresis (PFGE) was performed to determine the epidemiologic features.
Results
The imipenem non-susceptible rates were variable from 10% to 67%. Among 151 isolates carrying blaOXA-51-like, 75 isolates carried both blaOXA-51-like and ISAba1, and 25 isolates had both blaOXA-51-like, blaOXA-23-like, and ISAba1. Carbapenem MICs of both blaOXA-51-like and ISAba1-carrying isolates were higher than those with blaOXA-51-like only. Carbapenem MICs of blaOXA-23-like-carrying isolates were higher than those with both blaOXA-51-like and ISAba1. Both blaOXA-51-like and ISAba1-carrying isolates and blaOXA-51-like, blaOXA-23-like, and ISAba1-carrying isolates demonstrated higher hydrolysis activities in oxacillin and carbapenems. Most of the tested isolates were susceptible to tigecycline, and all of them were susceptible to colistin. Pulsed-field gel electrophoresis suggested that there had been several outbreaks of blaOXA-23-like and blaOXA-51-like-positive strains.
Conclusion
Carbapenem non-susceptible Acinetobacter isolates and OXA carbapenemase-producing isolates were prevalent. Dissemination of blaOXA-harboring isolates may make it difficult to treat infections due to carbapenem-resistant Acinetobacter spp. Further surveillance studies are required to prevent the spread of carbapenem resistance.
Go to : 
REFERENCES
1. Bergogne-Bérézin E and Towner KJ. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin Microbiol Rev. 1996; 9:148–65.

2. Lee K, Lim CH, Cho JH, Lee WG, Uh Y, Kim HJ, et al. High prevalence of ceftazidime-resistant Klebsiella pneumoniae and increase of imipenem-resistant Pseudomonas aeruginosa and Acinetobacter spp. in Korea: a KONSAR program in 2004. Yonsei Med J. 2006; 47:634–45.

3. Lee K, Jang SJ, Lee HJ, Ryoo N, Kim M, Hong SG, et al. Increasing prevalence of vancomycin-resistant Enterococcus faecium, expanded-spectrum cephalosporin-resistant Klebsiella pneumoniae, and imipenem-resistant Pseudomonas aeruginosa in Korea: KONSAR study in 2001. J Korean Med Sci. 2004; 19:8–14.
4. Poirel L and Nordmann P. Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clin Microbiol Infect. 2006; 12:826–36.
5. Bush K, Jacoby GA, Medeiros AA. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995; 39:1211–33.
6. Walther-Rasmussen J and H⊘oiby N. OXA-type carbapenemases. J Antimicrob Chemother. 2006; 57:373–83.
7. Naas T, Levy M, Hirschauer C, Marchandin H, Nordmann P. Outbreak of carbapenem-resistant Acinetobacter baumannii producing the carbapenemase OXA-23 in a tertiary care hospital of Papeete, French Polynesia. J Clin Microbiol. 2005; 43:4826–9.
8. Jeon BC, Jeong SH, Bae IK, Kwon SB, Lee K, Young D, et al. Investigation of a nosocomial outbreak of imipenem-resistant Acinetobacter baumannii producing the OXA-23 β-lactamase in Korea. J Clin Microbiol. 2005; 43:2241–5.
9. Héritier C, Dubouix A, Poirel L, Marty N, Nordmann P. A nosocomial outbreak of Acinetobacter baumannii isolates expressing the carbapenem-hydrolysing oxacillinase OXA-58. J Antimicrob Chemother. 2005; 55:115–8.
10. Turton JF, Ward ME, Woodford N, Kaufmann ME, Pike R, Livermore DM, et al. The role of ISAba1 in expression of OXA carbapenemase genes in Acinetobacter baumannii. FEMS Microbiol Lett. 2006; 258:72–7.

11. Lee K, Kim MN, Choi TY, Cho SE, Lee S, Whang DH, et al. Wide dissemination of OXA-type carbapenemases in clinical Acinetobacter spp. isolates from South Korea. Int J Antimicrob Agents. 2009; 33:520–4.

12. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. Sixteenth ed. Wayne, PA: CLSI;2006.
13. Lee K, Chong Y, Shin HB, Kim YA, Yong D, Yum JH. Modified Hodge and EDTA-disk synergy tests to screen metallo-β-lactamase-producing strains of Pseudomonas and Acinetobacter species. Clin Microbiol Infect. 2001; 7:88–91.
14. Lee K, Yum JH, Yong D, Lee HM, Kim HD, Docquier JD, et al. Novel acquired metallo-β-lactamase gene, blaSIM-1, in a class 1 integron from Acinetobacter baumannii clinical isolates from Korea. Antimicrob Agents Chemother. 2005; 49:4485–91.
15. Woodford N, Ellington MJ, Coelho JM, Turton JF, Ward ME, Brown S, et al. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents. 2006; 27:351–3.

16. Merkier AK and Centróon D. blaOXA-51-type β-lactamase genes are ubiquitous and vary within a strain in Acinetobacter baumannii. Int J Antimicrob Agents. 2006; 28:110–3.
17. Tsakris A, Ikonomidis A, Pournaras S, Spanakis N, Markogiannakis A. Carriage of OXA-58 but not of OXA-51 β-lactamase gene correlates with carbapenem resistance in Acinetobacter baumannii. J Antimicrob Chemother. 2006; 58:1097–9.

18. Segal H, Garny S, Elisha BG. Is ISAba-1 customized for Acinetobacter ? FEMS Microbiol Lett. 2005; 243:425–9.
Go to : 
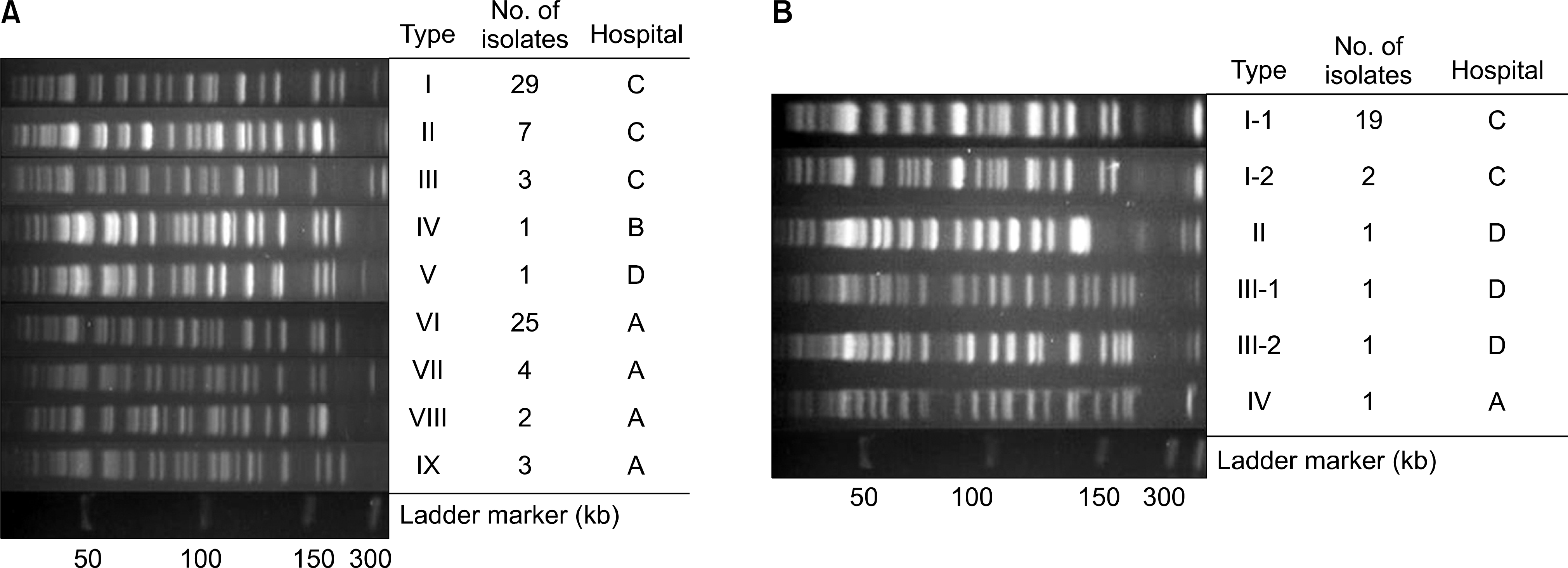 | Fig. 1.Pulsed-field gel electrophoresis (PFGE) patterns of SmaI-restricted genomic DNA of Acinetobacter spp. isolates. (A) Isolates having blaOXA-51 and ISAbaI. Lane I to IX, different clones from 4 hospitals; and (B) isolates having both blaOXA-51, blaOXA-23 and ISAbaI. Lane I-1 to IV, different clones from 4 hospitals; Molecular size (ladder marker) is given in kilobases. |
Table 1.
Primers used for detection and sequencing of the metallo-β-lactamase, OXA carbapenemase, and ISAba1 genes
Table 2.
Characteristics of OXA carbapenemase-producing Acinetobacter spp. by hospitals
Table 3.
Antimicrobial susceptibility of blaOXA-51-like-carrying Acinetobacter spp. isolates with or without ISAba1
Table 4.
Hydrolytic activities (relative %) of various OXA-carbapene-mase-producing strains




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download