Abstract
Background
Blood culture bottles with an antimicrobial removal system have been developed for patients treated with antibiotics. This study compared the ability of BACTEC Plus Aerobic/F bottles (Becton Dickinson, USA, BACTEC Plus) and BacT/Alert FA bottles (bio-Merieux Vitek, France) to effectively remove antimicrobials.
Methods
BACTEC Plus and BacT/Alert FA bottles were spiked with 5 mL human blood, peak therapeutic concentrations of 9 antimicrobials and 7 type strains. Three rounds of duplicate testing were completed per antimicrobial/strain combination and growth control without antimicrobials. The time to detection (TTD) and recovery rates for bacteria were compared for both systems.
Results
Overall, the BACTEC Plus and BacT/Alert FA recovered 76% (128/168) and 34% (57/168) of strains from test bottles, respectively. BACTEC Plus detected all of gram-positive bacteria except S. pneumoniae with ampicillin and ceftriaxone, but BacT/Alert FA detected 0∼50% of gram-positive bacteria except E. faecalis with vancomycin and methicillin-resistant S. aureus with oxacillin. In presence of cefepime, cefotaxime, cefoxitin and ceftriaxone, BACTEC Plus detected 33∼100% of gram-negative bacteria, but BacT/ Alert FA did not detect gram-negative bacteria at all. In presence of ciprofloxacin, BacT/Alert FA detected 100% of E. coli and K. pneumoniae compared with 33% of those for BACTEC Plus. Overall, TTD of BACTEC Plus was shorter than that of BacT/Alert FA except in detecting gram-negative bacteria with ciprofloxacin (P<0.05).
Go to : 
REFERENCES
1. Weinstein MP, Towns ML, Quartey SM, Mirrett S, Reimer LG, Parmigiani G, et al. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997; 24:584–602.

2. Cho YK, Kim DS, Choi SI, Lee HS. The influence of antimicrobial abuse to blood culture. Korean J Clin Microbiol. 1998; 1:63–7.
3. McGuire NM, Kauffman CA, Hertz CS, Kovach JM. Evaluation of the BACTEC antimicrobial removal system for detection of bacteremia. J Clin Microbiol. 1983; 18:449–51.

4. Moody JA, Fasching CE, Shanholtzer CJ, Gerding DN, Peterson LR. Evaluation of new blood culture processing systems. J Clin Microbiol. 1984; 20:351–6.

5. Courcol RJ, Durocher AV, Roussel-Delvallez M, Fruchart A, Martin GR. Routine evaluation of BACTEC NR-16A and NR-17A media. J Clin Microbiol. 1988; 26:1619–22.

6. Kelly MT, Roberts FJ, Henry D, Geere I, Smith JA. Clinical comparison of isolator and BACTEC 660 resin media for blood culture. J Clin Microbiol. 1990; 28:1925–7.

7. Tarrand JJ, Guillot C, Wenglar M, Jackson J, Lajeunesse JD, Rolston KV. Clinical comparison of the resin-containing BACTEC 26 Plus and the Isolator 10 blood culturing systems. J Clin Microbiol. 1991; 29:2245–9.

8. Weinstein MP, Mirrett S, Reimer LG, Wilson ML, Smith-Elekes S, Chuard CR, et al. Controlled evaluation of BacT/Alert standard aerobic and FAN aerobic blood culture bottles for detection of bacteremia and fungemia. J Clin Microbiol. 1995; 33:978–81.

9. McDonald LC, Fune J, Gaido LB, Weinstein MP, Reimer LG, Flynn TM, et al. Clinical importance of increased sensitivity of BacT/Alert FAN aerobic and anaerobic blood culture bottles. J Clin Microbiol. 1996; 34:2180–4.

10. Wilson ML, Mirrett S, Meredith FT, Weinstein MP, Scotto V, Reller LB. Controlled clinical comparison of BACTEC plus anaerobic/F to standard anaerobic/F as the anaerobic companion bottle to plus aerobic/F medium for culturing blood from adults. J Clin Microbiol. 2001; 39:983–9.

11. Pohlman JK, Kirkley BA, Easley KA, Basille BA, Washington JA. Controlled clinical evaluation of BACTEC Plus Aerobic/F and BacT/Alert Aerobic FAN bottles for detection of bloodstream infections. J Clin Microbiol. 1995; 33:2856–8.

12. Flayhart D, Borek AP, Wakefield T, Dick J, Carroll KC. Comparison of BACTEC PLUS blood culture media to BacT/Alert FA blood culture media for detection of bacterial pathogens in samples containing therapeutic levels of antibiotics. J Clin Microbiol. 2007; 45:816–21.

13. Viganò EF, Vasconi E, Agrappi C, Clerici P. Use of simulated blood cultures for time to detection comparison between BacT/ALERT and BACTEC 9240 blood culture systems. Diagn Microbiol Infect Dis. 2002; 44:235–40.

14. Viganò EF, Vasconi E, Agrappi C, Clerici P, Melloni P. Use of simulated blood cultures for antibiotic effect on time to detection of the two blood culture systems BacT/ALERT and BACTEC 9240. New Microbiol. 2004; 27:235–48.
15. Amsden GW. Tables of Antimicrobial Agent Pharmacology. Mandell GL, Bennett JE, editors. eds.Principles and Practice of Infectious Diseases. 6th ed.Philadelphia: Elservier Churchill Livingstone;2006. p. 635–700.

16. Lee GI, Hong KS, Kim OK. Results of blood cultures at Ewha Womans’ University Hospital in recent 5 years. Korean J Clin Pathol. 1988; 8:169–75.
17. Kim HK, Lee KW, Chong YS, Kwon OH, Kim JM, Kim DS. Blood culture results at the Severance Hospital during 1984-1993. Korean J Infect Dis. 1996; 28:156–66.
18. Kim MH, Lee WI, Lee HJ, Suh JT. Comparison of the nonra-diometric BACTEC NR-660 system and conventional blood culture system for the detection of bacteremia. Korean J Clin Pathol. 1994; 14:70–9.
19. Ko GA, Lee YS, Park CJ, Park SY, Cho HC, Lee KM. Evaluation of VITAL automated blood culture system. Korean J Clin Pathol. 1996; 16:556–62.
20. Lee DD, Lee SM, Choi JC, Lee EY, Chang CL. Evaluation of FAN-aerobic blood culture bottle in BacT/Alert3D system. Korean J Clin Microbiol. 2005; 8:148–52.
Go to : 
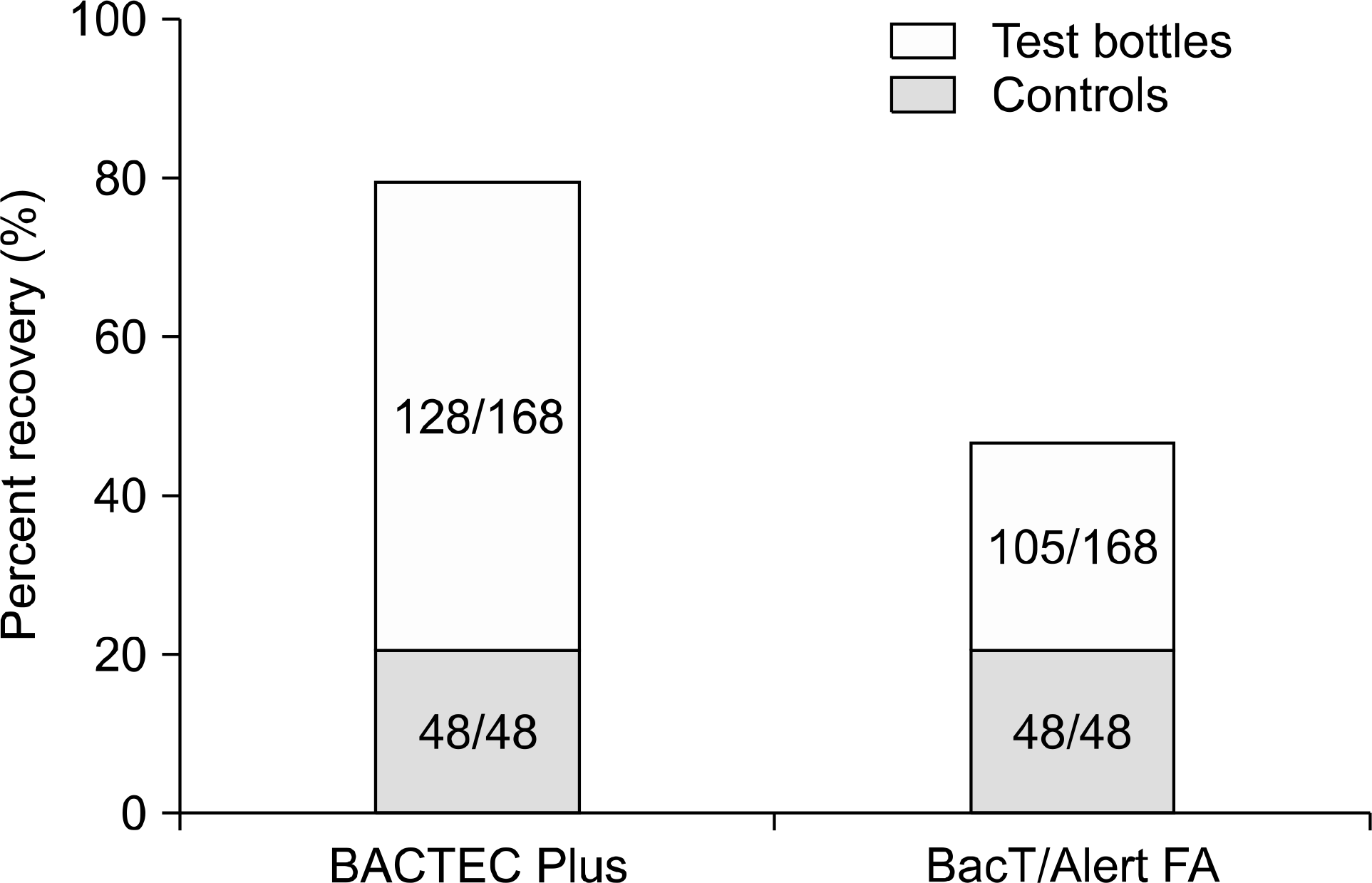 | Fig. 1.Overall recovery rates from growth controls and test bottles for the BACTEC Plus and BacT/Alert FA system. |
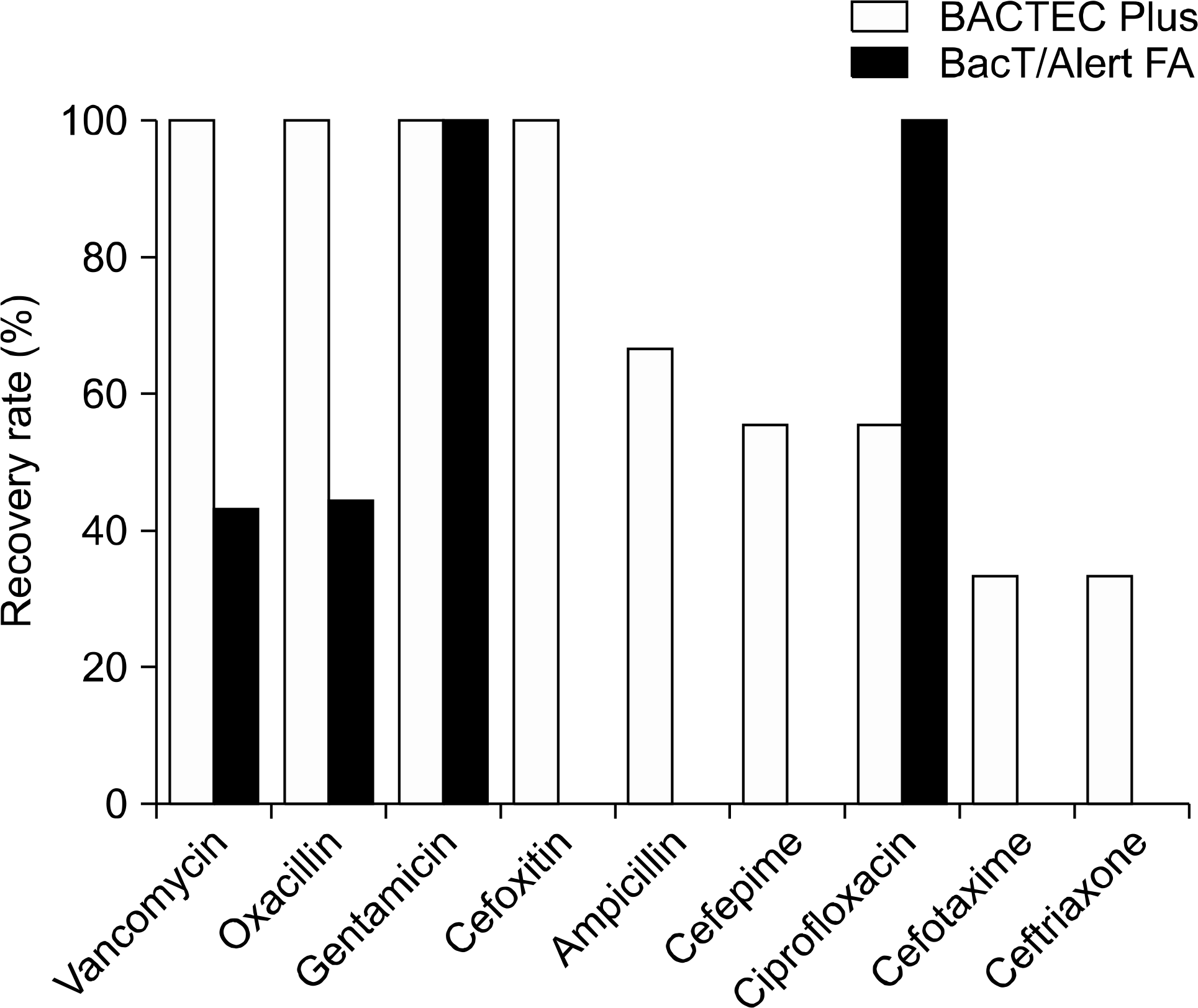 | Fig. 2.Overall recovery rates of organisms in BACTEC Plus Aerobic/F and BacT/Alert FA bottles containing antimicrobials. |
Table 1.
Combinations of strain/antimicrobials used in this study
Table 2.
Recovery rates (%) and time to detection (mean±SD) of gram-positive bacteria for BACTEC Plus and BacT/Alert FA at each antimicrobials
| Antimicrobials | No. (%) of growth positive bottles | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S. auureus | MRSA | MRSA-c | S. pneumoniae | E. faecalis | ||||||
| BACTEC | BacT/Alert | BACTEC | BacT/Alert | BACTEC | BacT/Alert | BACTEC | BacT/Alert | BACTEC | BacT/Alert | |
| VANN (RR) | 6 (100) | 2 (33) | 6 (100) | 1 (17) | 6 (100) | 3 (50) | 6 (100) | 1 (17) | 6 (100) | 6 (100) |
| TTD | 14.7±1.4 | 18.1±3.2 | 36.8±2.7 | 50.1±0.0 | 17.8±1.4 | 22.1±2.6 | 16.2±1.4 | 13.5±0.0 | 11.4±0.2∗ | 13.2±0.7∗ |
| AMPRR | NT | NT | NT | NT | NT | NT | 2 (33) | 0 (0) | 6 (100) | 0 (0) |
| TTD | NT | NT | NT | NT | NT | NT | 63.8±0.2 | No growth | 14.1±1.9 | No growth |
| OXAN (RR) | 6 (100) | 0 (0) | 6 (100) | 2 (33) | 6 (100) | 6 (100) | NT | NT | NT | NT |
| TTD | 21.9±3.1 | No growth | 39.8±7.4 | 34.5±1.9 | 18.1±4.3∗ | 24.2±2.7∗ | NT | NT | NT | NT |
| FOX N (RR) | 6 (100) | 0 (0) | NT | NT | NT | NT | 6 (100) | 0 (0) | NT | NT |
| TTD | 14.9±0.6 | No growth | NT | NT | NT | NT | 11.9±0.9 | No growth | NT | NT |
| CRON (RR) | NT | NT | NT | NT | NT | NT | 2 (33) | 0 (0%) | NT | NT |
| TTD | NT | NT | NT | NT | NT | NT | 64.5±0.0 | No growth | NT | NT |
| Ctrl N (RR) | 6 (100.0) | 6 (100.0) | 6 (100.0) | 6 (100.0) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) |
| TTD | 12.1±0.5∗ | 13.2±0.5∗ | 32.5±3.4† | 30.0±1.4 | 14.8±1.4† | 15.5±1.8† | 10.4±0.5∗ | 12.3±0.6∗ | 10.8±0.7∗ | 13.5±0.5∗ |
Abbreviations: BACTEC, BACTEC Plus Aerobic/F; BacT/Alert, Bact/Alert FA; VAN, vancomycin; AMP, ampicillin; OXA, oxacillin; FOX, cefoxitin; CRO, ceftriaxone; Ctrl, control; MRSA, methicillin resistence S. aureus; MRSA-c, clinical isolate of MRSA; N, number of growth positive bottles; RR, recovery rate; TTD, time to detection (hours); NT, not tested.
Table 3.
Recovery rates (%) and time to detection (mean±SD) of gram-negative bacteria for BACTEC Plus and BacT/Alert FA at each antimicrobials
| Antimicrobials | No. (%) of growth positive bottles | ||||||
|---|---|---|---|---|---|---|---|
| E. coli | K. pneumoniae | P. aeruginosa | |||||
| BACTEC | BacT/Alert | BACTEC | BacT/Alert | BACTEC | BacT/Alert | ||
| Gentamicin | N (RR) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) |
| TTD | 10.7±0.3∗ | 11.6±0.1∗ | 10.6±0.3∗ | 12.0±0.2∗ | 14.0±0.9∗ | 15.1±0.7∗ | |
| Cefepime | N (RR) | 2 (33) | 0 (0) | 2 (33) | 0 (0) | 6 (100) | 0 (0) |
| TTD | 39.8±0.6 | No growth | 40.0±0.4 | No growth | 24.9±6.4 | No growth | |
| Ciprofloxacin | N (RR) | 2 (33%) | 6 (100%) | 2 (33) | 6 (100) | 6 (100) | 6 (100) |
| TTD | 39.7±0.5 | 13.6±1.1 | 39.5±0.2 | 12.3±0.4 | 21.6±11.6† | 15.2±0.7† | |
| Cefotaxime | N (RR) | 2 (33) | 0 (0) | 2 (33) | 0 (0) | NT | NT |
| TTD | 39.2±0.2 | No growth | 39.4±0.5 | No growth | NT | NT | |
| Cefoxitin | N (RR) | 6 (100) | 0 (0) | 6 (100) | 0 (0) | NT | NT |
| TTD | 10.5±0.8 | No growth | 10.6±0.3 | No growth | NT | NT | |
| Ceftriaxone | N (RR) | 2 (33) | 0 (0) | 2 (33) | 0 (0) | NT | NT |
| TTD | 39.4±0.5 | No growth | 40.4±0.3 | No growth | NT | NT | |
| Control | N (RR) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) |
| TTD | 10.7±0.2∗ | 11.7±0.1∗ | 10.4±0.4∗ | 12.0±0.3∗ | 13.9±1.4† | 15.2±0.5† | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download