Abstract
Background
Novel swine influenza (H1N1) was first identified in Mexico in April 2009. Because of its high infectivity and worldwide distribution, a rapid and efficient screening test is necessary. Here we evaluated the usefulness of a rapid antigen test currently in use, compared to realtime RT-PCR (rRT-PCR) as a screening test for detection of novel swine influenza (H1N1).
Methods
A total of 1,228 patients who visited Hallym University Kangdong Sacred Heart Hospital with influenza-like illness between 14 August 2009 and 30 September 2009, and were tested by both rapid antigen and rRT-PCR tests, were enrolled in this study.
Results
Sensitivity, specificity, predictive value of a positive test, and predictive value of a negative test for the rapid antigen test were 30.5%, 99.2%, 86.4% and 90.1%, respectively. Fifty-one (4.2%) patients were positive for both rapid antigen test and rRT-PCR, and 1,053 (85.7%) were negative for both rapid antigen test and rRT-PCR. A total of 124 (10.1%) patients showed a discrepancy between the two tests. Among them, 116 (9.4%) were only positive for rRT-PCR and 8 (0.7%) were only positive for the rapid antigen test. The latter 8 patients all showed negative H1/M2 results in rRT-PCR. There were significant differences in detection rates of the rapid antigen test between different H1 Ct (threshold cycle) interval groups and for different age groups (P <0.05).
Go to : 
REFERENCES
1. Kim SH, Huh JH, Bae SY, Kim JS, Yoon SY, Lim CS, et al. Epidemiology of respiratory viral infection in 2004-2006. Korean J Lab Med. 2006; 26:351–7.

2. Lee WG, Lee HK, Kim HJ, Chung JK, Lee EH, Moon HR. Evaluation of a rapid antigen test for detection of influenza virus. Korean J Clin Microbiol. 2004; 7:119–23.
3. Petric M, Comanor L, Petti CA. Role of the laboratory in diagnosis of influenza during seasonal epidemics and potential pandemics. J Infect Dis. 2006; 194(Suppl 2):S98–110.

4. Peiris JS, Poon LL, Guan Y. Emergence of a novel swine-origin influenza A virus (S-OIV) H1N1 virus in humans. J Clin Virol. 2009; 45:169–73.
5. Gatherer D. The 2009 H1N1 influenza outbreak in its historical context. J Clin Virol. 2009; 45:174–8.

6. World Health Organization. WHO web sites on disease outbreak news. Pandemic (H1N1) 2009 - update 71.http://www.who.int/csr/don/2009_10_23/en. [Online] (last visited on 17 October 2009).
7. Panning M, Eickmann M, Landt O, Monazahian M, Olschläger S, Baumgarte S, et al. Detection of influenza A(H1N1)v virus by realtime RT-PCR. Euro Surveill. 2009; 14:pii: 19329.

8. Vasoo S, Stevens J, Singh K. Rapid antigen tests for diagnosis of pandemic (Swine) influenza A/H1N1. Clin Infect Dis. 2009; 49:1090–3.

9. Rahman M, Vandermause MF, Kieke BA, Belongia EA. Performance of Binax NOW Flu A and B and direct fluorescent assay in comparison with a composite of viral culture or reverse transcription polymerase chain reaction for detection of influenza infection during the 2006 to 2007 season. Diagn Microbiol Infect Dis. 2008; 62:162–6.

10. Song SH and Kim EC. Rapid diagnosis of respiratory virus infection. J Lab Med Qual Assur. 2007; 29:207–10.
11. Kim JS, Choi HJ, Ahn YM, Hwang YO. Clinical usefulness of rapid antigen test on the diagnosis of influenza. Korean J Pediatr. 2005; 48:1348–53.
12. Chan KH, Lai ST, Poon LL, Guan Y, Yuen KY, Peiris JS. Analytical sensitivity of rapid influenza antigen detection tests for swine-origin influenza virus (H1N1). J Clin Virol. 2009; 45:205–7.

13. Cheng CK, Cowling BJ, Chan KH, Fang VJ, Seto WH, Yung R, et al. Factors affecting QuickVue Influenza A+B rapid test performance in the community setting. Diagn Microbiol Infect Dis. 2009; 65:35–41.
14. Ruest A, Michaud S, Deslandes S, Frost EH. Comparison of the Directigen flu A+B test, the QuickVue influenza test, and clinical case definition to viral culture and reverse transcription-PCR for rapid diagnosis of influenza virus infection. J Clin Microbiol. 2003; 41:3487–93.

15. Centers for Disease Control and Prevention (CDC). Evaluation of rapid influenza diagnostic tests for detection of novel influenza A (H1N1) Virus - United States, 2009. MMWR Morb Mortal Wkly Rep. 2009; 58:826–9.
16. Kang JO, Kim EC, Lee KM, Lee NY, Lee CK. Surveillance for respiratory virus testing situation in Korea and epidemiology for the respiratory viruses detected in 5 university hospitals: report from virus study group. Korean J Clin Microbiol. 2007; 10:102–8.
17. Chan KH, Lam SY, Puthavathana P, Nguyen TD, Long HT, Pang CM, et al. Comparative analytical sensitivities of six rapid influenza A antigen detection test kits for detection of influenza A subtypes H1N1, H3N2 and H5N1. J Clin Virol. 2007; 38:169–71.

18. Yoo Y, Sohn JW, Park DW, Kim JY, Shin HK, Lee Y, et al. Clinical evaluation of the SD Bioline influenza virus antigen test for rapid detection of influenza viruses A and B in children and adults during the influenza season. Clin Vaccine Immunol. 2007; 14:1050–2.

19. Mai LQ, Hien PT, Hang NL, Oh JS, Ha GW, Kwon JA, et al. Evaluation of two lateral-flow chromatographic membrane lmmu-noassays for rapid detection of influenza virus in limited respiratory specimens. J Lab Med Qual Assur. 2005; 27:243–9.
20. Chan KH, Maldeis N, Pope W, Yup A, Ozinskas A, Gill J, et al. Evaluation of the directigen FluA+B test for rapid diagnosis of influenza virus type A and B infections. J Clin Microbiol. 2002; 40:1675–80.

21. World Health Organization. WHO recommendations on the use of rapid testing for influenza diagnosis. http://www.who.int/csr/disease/avian_influenza/guidelines/RapidTestInfluenza_web.pdf. (last visited on July 2005).
Go to : 
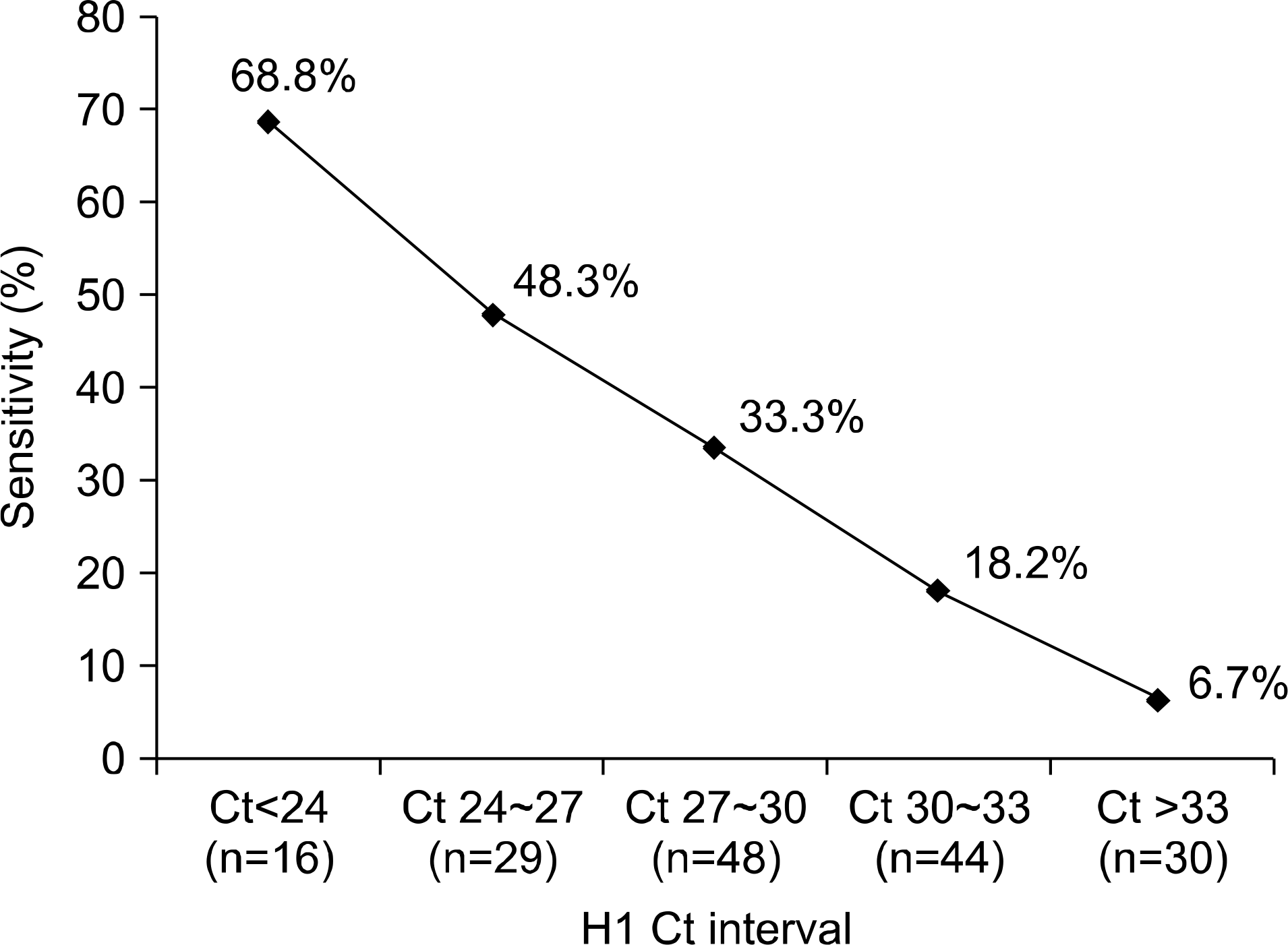 | Fig. 1.Comparison of rapid antigen test sensitivity between different H1 Ct∗ interval groups among novel swine influenza A (H1N1) patients confirmed by realtime RT-PCR. ∗Ct, threshold cycle. |
Table 1.
Comparison of rapid antigen test and realtime RT-PCR for detection of novel swine influenza A (H1N1)
| Real-time RT-PCR | Rapid antigen test∗ | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 51 (4.2%) | 116 (9.4%) | 167 (13.6%) |
| Negative | 8 (0.7%) | 1,053 (85.7%) 1 | 1,061 (86.4%) |
| Total | 59 (4.8%) | 1,169 (95.2%) 1 | 1,228 (100.0%) |
Table 2.
Comparison of rapid antigen test results between different age groups among novel swine influenza A (H1N1) patients confirmed by realtime RT-PCR
| Age (year) | Rapid antigen test∗ | ||
|---|---|---|---|
| Positive | Negative | Total | |
| <13 | 20 (44.4%) | 25 (55.6%) | 45 |
| 13∼29 | 29 (26.9%) | 25 (55.6%) | 108 |
| ≥30 | 2 (14.3%) | 116 (69.5%) | 14 |
| Total | 51 (30.5%) | 116 (69.5%) | 167 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download