Abstract
Background
CLSI provides a guideline only for a agar dilution method of testing clarithromycin susceptibility for Helicobacter pylori. This study was to evaluate a disk diffusion method for clarithromycin and amoxicillin.
Methods
One hundred and forty clinical isolates of H. pylori isolated from May 2005 to May 2007 were tested by the CLSI agar dilution method and a disk diffusion method using 2μg (2CLR) and 15μg (15CLR) clarithromycin disks and 2μg (2AMX) and 10μg (10AMX) amoxicillin disks. The interpretation criteria used for the disk diffusion method were established by linear regression and error rate-bounded method for disk diffusion zone of inhibition (DDZ) compared to MIC.
Results
Resistance and intermediate rates to clarithromycin were 21.4% and 1.4%, respectively. A number of isolates with MIC 0.5, 1, and 2 (μg/mL) to amoxicillin were 7, 2, and 1, respectively. For 2CLR and 15CLR, the coefficients of determination (R2) between MIC and DDZ were 0.931 and 0.923 (P< 0.001), respectively, and the criteria for resistance/ susceptibility were 12/28 mm for 2CLR and 23/39 mm for 15CLR. For 2AMX and 10AMX, the R2 between MIC and DDZ were 0.478 and 0.421 (P< 0.001), respectively, and the criteria for resistance with breakpoint of 2μg/mL were 21 mm for 2AMX and 32 mm for 10AMX. All isolates had DDZ<60 mm with 2CLR and 2AMX, but 61.4% and 75.7% of the isolates had DDZ<60 mm with 15CLR and 10AMX, respectively.
Go to : 
REFERENCES
1. NIH consensus conference. Helicobacter pylori in peptic ulcer disease. NIH consensus development panel on Helicobacter pylori in peptic ulcer disease. JAMA. 1994; 272:65–9.
2. Blaser MJ. Hypothesis: the changing relationships of Helicobacter pylori and humans: implications for health and disease. J Infect Dis. 1999; 179:1523–30.
3. Kim JG. Treatment of Helicobacter pylori infection. Korean J Gastroenterol. 2005; 46:172–80.
4. Sung H, Chung HJ, Kim MN, Lee GH. Clinical usefulness of antimicrobial susceptibility test for Helicobacter pylori. Korean J Lab Med. 2006; 26:179–84.
5. Malfertheiner P, Mégraud F, O'Morain C, Hungin AP, Jones R, Axon A, et al. Current concepts in the management of Helicobacter pylori infection-the Maastricht 2-2000 Consensus Report. Aliment Pharmacol Ther. 2002; 16:167–80.
6. Eun CS, Han DS, Park JY, Jeon YC, Hahm JS, Kim KS, et al. Changing pattern of antimicrobial resistance of Helicobacter pylori in Korean patients with peptic ulcer diseases. J Gastroenterol. 2003; 38:436–41.
7. Bang SY, Han DS, Eun CS, Kim JE, Ahn SB, Sohn JH, et al. Changing patterns of antibiotic resistance of Helicobacter pylori in patients with peptic ulcer disease. Korean J Gastroenterol. 2007; 50:356–62.
8. Kim JM. Antibiotic resistance of Helicobacter pylori isolated from Korean patients. Korean J Gastroenterol. 2006; 47:337–49.
9. Kim JM, Kim JS, Kim N, Kim SG, Jung HC, Song IS. Comparison of primary and secondary antimicrobial minimum inhibitory concentrations for Helicobacter pylori isolated from Korean patients. Int J Antimicrob Agents. 2006; 28:6–13.
10. National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing. Ninth informational supplement. M100-S9. Wayne, PA; NCCLS,. 1999.
11. Mégraud F, Lehn N, Lind T, Bayerdörffer E, O'Morain C, Spiller R, et al. Antimicrobial susceptibility testing of Helicobacter pylori in a large multicenter trial: the MACH 2 study. Antimicrob Agents Chemother. 1999; 43:2747–52.
12. Kang JO, Han D, Choi TY. Evaluation of four methods for antimicrobial susceptibility testing of Helicobacter pylori in routine practice. Korean J Clin Microbiol. 2005; 8:82–9.
13. Lang L, Garcia F. Comparison of E-test and disk diffusion assay to evaluate resistance of Helicobacter pylori isolates to amoxicillin, clarithromycin, metronidazole and tetracycline in Costa Rica. Int J Antimicrob Agents. 2004; 24:572–7.
14. Hachem CY, Clarridge JE, Reddy R, Flamm R, Evans DG, Tanaka SK, et al. Antimicrobial susceptibility testing of Helicobacter pylori. Comparison of E-test, broth microdilution, and disk diffusion for ampicillin, clarithromycin, and metronidazole. Diagn Microbiol Infect Dis. 1996; 24:37–41.
15. Boyanova L, Mentis A, Gubina M, Rozynek E, Gosciniak G, Kalenic S, et al. The status of antimicrobial resistance of Helicobacter pylori in eastern Europe. Clin Microbiol Infect. 2002; 8:388–96.
16. Mishra KK, Srivastava S, Garg A, Ayyagari A. Antibiotic susceptibility of Helicobacter pylori clinical isolates: comparative evaluation of disk-diffusion and E-test methods. Curr Microbiol. 2006; 53:329–34.
17. Metzler CM, DeHaan RM. Susceptibility tests of anaerobic bacteria: statistical and clinical considerations. J Infect Dis. 1974; 130:588–94.

18. Iovene MR, Romano M, Pilloni AP, Giordano B, Montella F, Caliendo S, et al. Prevalence of antimicrobial resistance in eighty clinical isolates of Helicobacter pylori. Chemotherapy. 1999; 45:8–14.
19. Weiss K, Laverdière M, Restieri C. Comparison of activity of 10 antibiotics against clinical strains of Helicobacter pylori by three different techniques. Can J Gastroenterol. 1998; 12:181–5.
20. Midolo PD, Bell JM, Lambert JR, Turnidge JD, Grayson ML. Antimicrobial resistance testing of Helicobacter pylori: a comparison of Etest and disk diffusion methods. Pathology. 1997; 29:411–4.
21. Smith C, Perkins J, Tompkins D. Comparison of Etest and disc diffusion for detection of antibiotic resistance in Helicobacter pylori. PHLS Microbiol Dig. 1997; 14:21–3.
22. Grignon B, Tankovic J, Mégraud F, Glupczynski Y, Husson MO, Conroy MC, et al. Validation of diffusion methods for macrolide susceptibility testing of Helicobacter pylori. Microb Drug Resist. 2002; 8:61–6.
23. McNulty C, Owen R, Tompkins D, Hawtin P, McColl K, Price A, et al. Helicobacter pylori susceptibility testing by disc diffusion. J Antimicrob Chemother. 2002; 49:601–9.
24. Clinical and Laboratory Standards Institute. Development of in vitro susceptibility testing criteria and quality control parameters. M23-A3. Wayne, PA; CLSI,. 2008.
25. Kim JM, Kim JS, Jung HC, Kim N, Kim YJ, Song IS. Distribution of antibiotic MICs for Helicobacter pylori strains over a 16-year period in patients from Seoul, South Korea. Antimicrob Agents Chemother. 2004; 48:4843–7.
26. Franzin L, Pennazio M, Cabodi D, Paolo Rossini F, Gioannini P. Clarithromycin and amoxicillin susceptibility of Helicobacter pylori strains isolated from adult patients with gastric or duodenal ulcer in Italy. Curr Microbiol. 2000; 40:96–100.
27. Kim JJ, Reddy R, Lee M, Kim JG, El-Zaatari FA, Osato MS, et al. Analysis of metronidazole, clarithromycin and tetracycline resistance of Helicobacter pylori isolates from Korea. J Antimicrob Chemother. 2001; 47:459–61.
28. Alarcón T, Domingo D, López-Brea M. Antibiotic resistance problems with Helicobacter pylori. Int J Antimicrob Agents. 1999; 12:19–26.
Go to : 
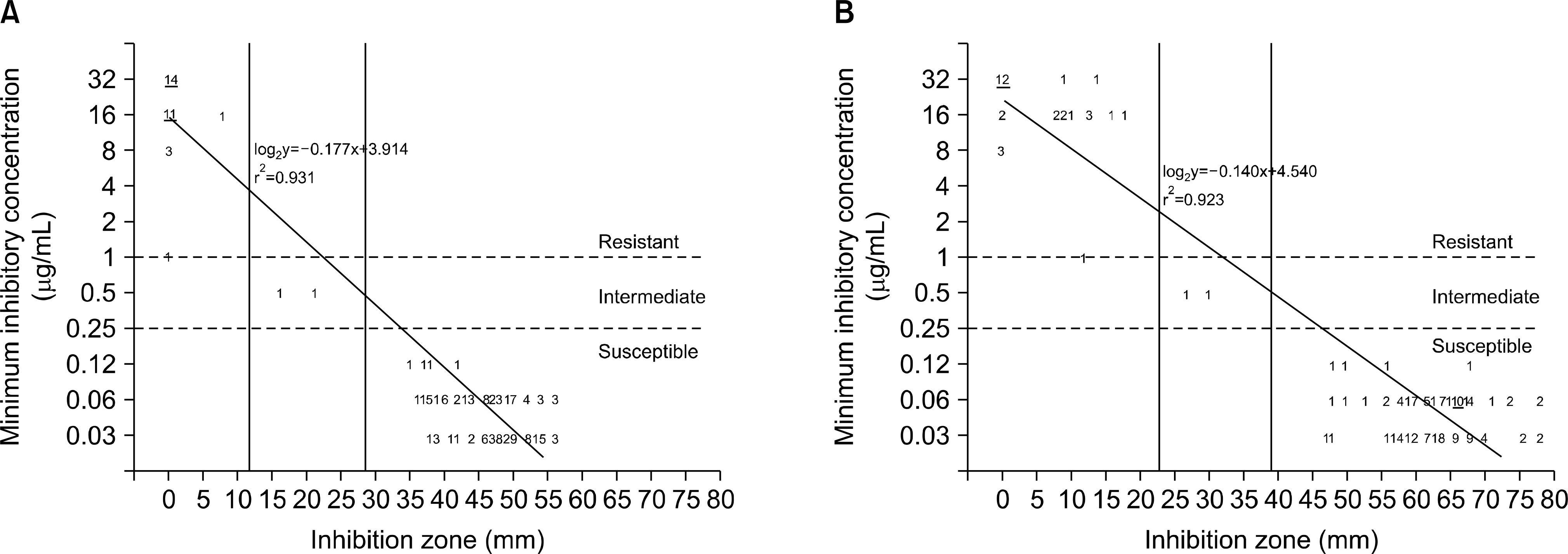 | Fig. 1.Error rate-bounded analyses of agar dilution MIC versus disk diffusion zone diameter for clarithromycin against 140 H. pylori clinical isolates. There was no major or minor error with resistance and susceptibility criteria of 12 mm and 28 mm for 2μg disk (A) and 23 mm and 34 mm for 15μg disk (B). The number of isolates more than 10 was indicated with underline. |
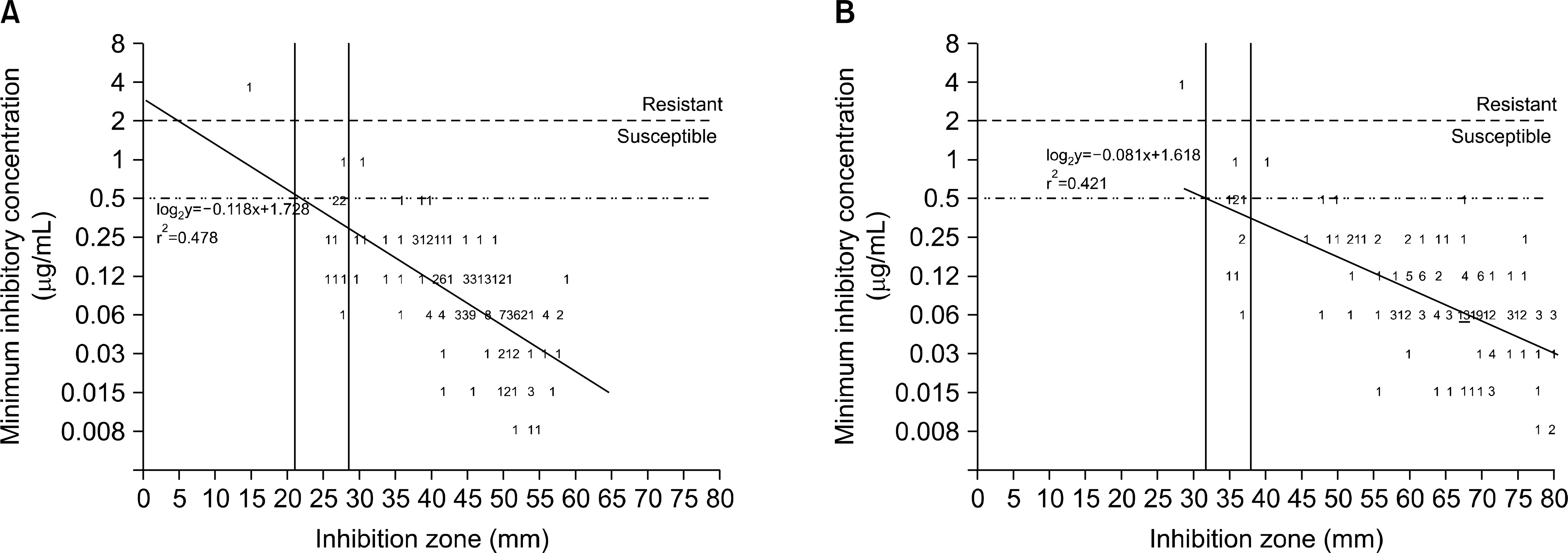 | Fig. 2.Error rate-bounded analyses of agar dilution MIC versus disk diffusion zone diameter for amoxicillin against 140 H. pylori clinical isolates. Dotted line and dotdash lines were for the breakpoint of 2μg/mL and 0.5μg/mL, respectively. At the resistance breakpoint of 2μg/mL, there was no major or minor error when 21 mm for 2μg disk (A) and 32 mm for 10μg disk (B) was applied for resistance criteria. The number of isolates more than 10 was indicated with underline. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download