Abstract
Background
Accurate detection of organisms producing extended-spectrum β-lactamase (ESBL) and AmpC β-lactamase is very important for treatment of patients. However, unlike the ESBL confirmatory test, there are no guidelines for detection of organisms producing AmpC β-lactamase. We evaluated a detection method using boronic acid (BA) for ESBL and AmpC β-lactamase.
Methods
Clinical isolates of Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, and Proteus mirabilis showing intermediate resistance or resistance to cefoxitin (FOX) or positive for ESBL were tested. A ≥5 mm increase in zone diameter of ceftazidime/clavulanic acid/BA (CAZ/CA/BA) and/or cefotaxime/clavulanic acid/BA (CTX/CA/BA) versus CAZ/BA and/or CTX /BA was considered positive for ESBL. Likewise, a ≥5 mm increase in zone diameter of FOX/BA and/or cefotetan/BA (CTT/BA) versus FOX and/or CTT alone was considered positive for AmpC β-lactamase.
Results
Among 622 clinical isolates, ESBL positive rates by the CLSI ESBL confirmatory test or by the BA method were 18.1% or 18.4% for E. coli, 38.3% or 40.4% for K. pneumoniae, 8.7% or 8.7% for K. oxytoca, and 14.8% or 14.8% for P. mirabilis, respectively. AmpC β-lactamase positive rates using the BA method were 3.7% for E. coli, 33.3% for K. pneumoniae, 0% for K. oxytoca, and 7.4% for P. mirabilis. The detection rates of coproducing ESBL and AmpC β-lactamase were 2.4% in E. coli 27.1% in K. pneumoniae, and 3.7% in P. mirabilis.
Go to : 
REFERENCES
1. Uh Y, Kim HY, et al. Antimicrobial Agents and Antimicrobial Susceptibility Test. 1st ed.Paju: KIS;2007. p. 118–9.
2. Beesley T, Gascoyne N, Knott-Hunziker V, Petursson S, Waley SG, Jaurin B, et al. The inhibition of class C β-lactamases by boronic acids. Biochem J. 1983; 209:229–33.

3. Brenwald NP, Jevons G, Andrews J, Ang L, Fraise P. Disc methods for detecting AmpC β-lactamase-producing clinical isolates of Escherichia coli and Klebsiella pneumoniae. J Antimicrob Chemother. 2005; 56:600–1.
4. Coudron PE, Moland ES, Thomson KS. Occurrence and detection of AmpC β-lactamases among Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis isolates at a veterans medical center. J Clin Microbiol. 2000; 38:1791–6.
5. Yagi T, Wachino J, Kurokawa H, Suzuki S, Yamane K, Doi Y, et al. Practical methods using boronic acid compounds for identification of class C β-lactamase-producing Klebsiella pneumoniae and Escherichia coli. J Clin Microbiol. 2005; 43:2551–8.
6. Song W, Bae IK, Lee YN, Lee CH, Lee SH, Jeong SH. Detection of extended-spectrum β-lactamases by using boronic acid as an AmpC β-lactamase inhibitor in clinical isolates of Klebsiella spp. and Escherichia coli. J Clin Microbiol. 2007; 45:1180–4.
7. Song W, Jeong SH, Kim JS, Kim HS, Shin DH, Roh KH, et al. Use of boronic acid disk methods to detect the combined expression of plasmid-mediated AmpC β-lactamases and extended-spectrum β-lactamases in clinical isolates of Klebsiella spp., Salmonella spp., and Proteus mirabilis. Diagn Microbiol Infect Dis. 2007; 57:315–8.
8. Uh Y, Son JS, Hwang GY, Jang IH, Yoon KJ, Seo DM. Microplate identification system of Enterobacteriaceae. Korean J Clin Microbiol. 1999; 2:135–43.
9. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 16th informational supplement. Document M100-S16. Wayne, PA; CLSI,. 2006.
10. Bauernfeind A, Stemplinger I, Jungwirth R, Giamarellou H. Characterization of the plasmidic β-lactamase CMY-2, which is responsible for cephamycin resistance. Antimicrob Agents Chemother. 1996; 40:221–4.
11. Queenan AM, Jenkins S, Bush K. Cloning and biochemical characterization of FOX-5, an AmpC-type plasmid-encoded β-lactamase from a New York City Klebsiella pneumoniae clinical isolate. Antimicrob Agents Chemother. 2001; 45:3189–94.
12. Walther-Rasmussen J, H⊘iby N. Plasmid-borne AmpC β-lactamases. Can J Microbiol. 2002; 48:479–93.

13. Bauernfeind A, Hohl P, Schneider I, Jungwirth R, Frei R. Escherichia coli producing a cephamycinase (CMY-2) from a patient from the Libyan-Tunisian border region. Clin Microbiol Infect. 1998; 4:168–70.
14. Coudron PE, Hanson ND, Climo MW. Occurrence of extended-spectrum and AmpC beta-lactamases in bloodstream isolates of Klebsiella pneumoniae: isolates harbor plasmid-mediated FOX-5 and ACT-1 AmpC beta-lactamases. J Clin Microbiol. 2003; 41:772–7.
15. Bradford PA, Urban C, Mariano N, Projan SJ, Rahal JJ, Bush K. Imipenem resistance in Klebsiella pneumoniae is associated with the combination of ACT-1, a plasmid-mediated AmpC β-lactamase, and the loss of an outer membrane protein. Antimicrob Agents Chemother. 1997; 41:563–9.
16. Nadjar D, Rouveau M, Verdet C, Donay L, Herrmann J, Lagrange PH, et al. Outbreak of Klebsiella pneumoniae producing transferable AmpC-type β-lactamase (ACC-1) originating from Hafnia alvei. FEMS Microbiol Lett. 2000; 187:35–40.
17. Coudron PE. Inhibitor-based methods for detection of plasmid-mediated AmpC β-lactamases in Klebsiella spp., Escherichia coli, and Proteus mirabilis. J Clin Microbiol. 2005; 43:4163–7.
18. Song W, Kim JS, Kim HS, Yong D, Jeong SH, Park MJ, et al. Increasing trend in the prevalence of plasmid-mediated AmpC β-lactamases in Enterobacteriaceae lacking chromosomal ampC gene at a Korean university hospital from 2002 to 2004. Diagn Microbiol Infect Dis. 2006; 55:219–24.
19. Lee K, Hong SG, Park YJ, Lee HS, Song W, Jeong J, et al. Evaluation of phenotypic screening methods for detecting plasmid-mediated AmpC β-lactamases-producing isolates of Escherichia coli and Klebsiella pneumoniae. Diagn Microbiol Infect Dis. 2005; 53:319–23.
20. Black JA, Moland ES, Thomson KS. AmpC disk test for detection of plasmid-mediated AmpC β-lactamases in Enterobacteriaceae lacking chromosomal AmpC β-lactamases. J Clin Microbiol. 2005; 43:3110–3.
21. Pérez-Pérez FJ, Hanson ND. Detection of plasmid-mediated AmpC β-lactamase genes in clinical isolates by using multiplex PCR. J Clin Microbiol. 2002; 40:2153–62.

22. Smith Moland E, Hanson ND, Herrera VL, Black JA, Lockhart TJ, Hossain A, et al. Plasmid-mediated, carbapenem-hydrolysing β-lactamase, KPC-2, in Klebsiella pneumoniae isolates. J Antimicrob Chemother. 2003; 51:711–4.
23. Jeong SH, Song W, Park MJ, Kim JS, Kim HS, Bae IK, et al. Boronic acid disk tests for identification of extended-spectrum β-lactamase production in clinical isolates of Enterobacteriaceae producing chromosomal AmpC β-lactamases. Int J Antimicrob Agents. 2008; 31:467–71.
Go to : 
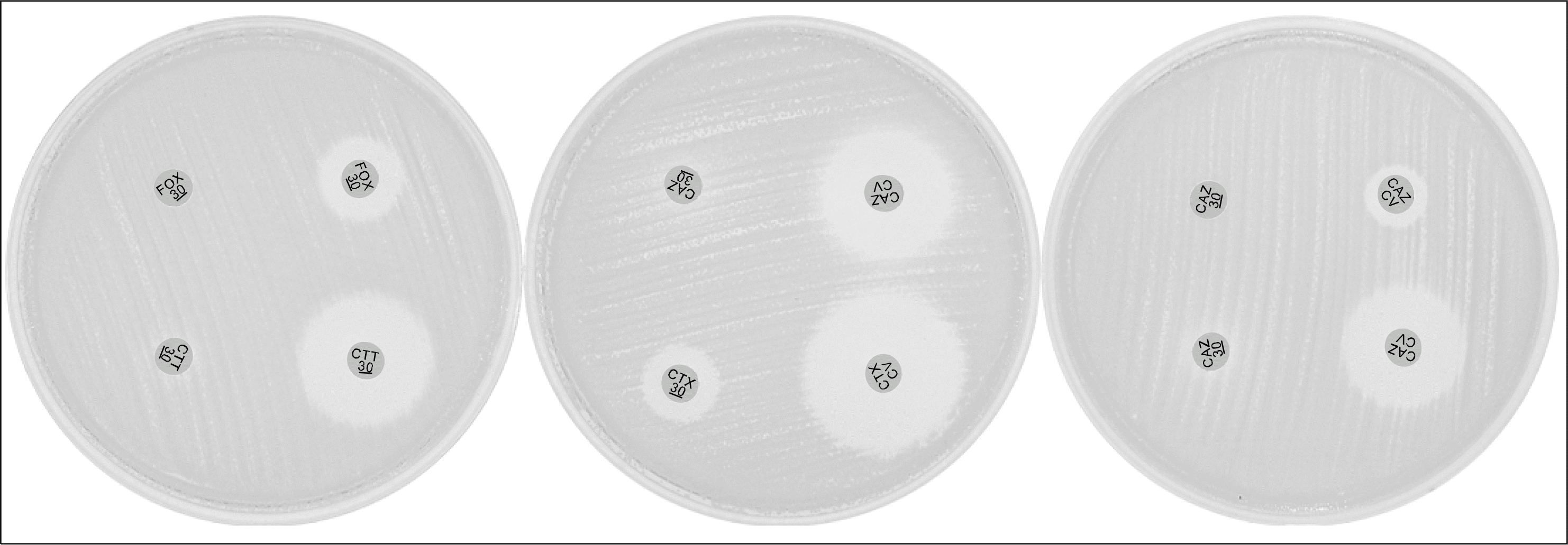 | Fig. 1.Representative results using the Clinical and Laboratory Standards Institute extended-spectrum β-lactamase (ESBL) confirmatory test and AmpC disk test without and with boronic acid (two disks positioned in right side on media). Abbreviations: FOX, cefoxitin; CAZ, ceftazidime; CV, clavulanic acid; CTX, cefotaxime; CTX, cefotaxime. Left media shows AmpC positive, center media shows ESBL positive, and right media shows that isolate is negative in CLSI method and positive in boronic acid disk. |
Table 1.
Comparison of CLSI ESBL confirmatory tests in Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, and Proteus mirabilis
Table 2.
Comparative results in E. coli, K. pneumoniae, K. oxytoca and P. mirabilis by combination with the boronic acid
Table 3.
Prevalence of AmpC and ESBL in E. coli, K. pneumoniae, K. oxytoca and P. mirabilis by combination with the boronic acid
Table 4.
Antimicrobial susceptibilities of E. coli, K. pneumoniae, K. oxytoca, and P. mirabilis according to the presence of AmpC and/or ESBL
Abbreviations: ESBL, extended-spectrum β-lactamase; SXT, cotrimoxazole; CIP, ciprofloxacin; AMK, amikacin; GM, gentamicin; TOB, tobramycin; MAN, cefamandole; CFS, cefoperazone/sulbactam; PPT, piperacillin/tazobactam; ATM, aztreonam; CTX, cefotaxime; CAZ, ceftazidime; FEP, cefepime; FOX, cefoxitin; CTT, cefotetan; IPM, imipenem.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download