Abstract
Background
Group B Streptococcus (Streptococcus agalactiae, GBS) is a major cause of severe infections in neonates, including bacteremia, pneumonia, and meningitis, and is generally vertically transmitted from a colonized, pregnant woman to her infant. Penicillin is the drug of choice to treat GBS infections, because GBS strains are uniformly susceptible to penicillin. Recently, however, penicillin resistant GBS strains have been reported and the rates of erythromycin and clindamycin resistance have increased. We evaluated the perineal colonization rates and antimicrobial susceptibility of GBS strains isolated from pregnant and non-pregnant women.
Methods
The antibiotic susceptibilities of a total of 180 GBS strains isolated from two university hospitals and one reference laboratory between May 2008 and January 2009 were determined using disk diffusion and broth microdilution methods. The presence of erythromycin resistance genes was confirmed by PCR.
Results
The average colonization rate of pregnant women was 5.5%. The overall colonization rates of pregnant and non-pregnant women ranged between 5.5% and 7.5%. All 180 GBS strains were susceptible to penicillin. Fifty strains (27.8%) were resistant to erythromycin, whereas 78 (41.1%) were resistant to clindamycin. The ermB gene was identified in 40 isolates and 44 isolates had constitutive macrolide-lincosamide-streptogramin B resistance phenotypes.
REFERENCES
1. Uh Y, Kwon JY, Jang IH, Yoon KJ, Kim HG. Colonization rate of Group B Streptococcus in pregnant women and neonates. Korean J Clin Pathol. 1994; 14:447–53.
2. Kimura K, Suzuki S, Wachino J, Kurokawa H, Yamane K, Shibata N, et al. First molecular characterization of Group B streptococci with reduced penicillin susceptibility. Antimicrob Agents Chemother. 2008; 52:2890–7.

3. Castor ML, Whitney CG, Como-Sabetti K, Facklam RR, Ferrieri P, Bartkus JM, et al. Antibiotic resistance patterns in invasive group B streptococcal isolates. Infect Dis Obstet Gynecol. 2008; 2008:727505.

4. Kimura K, Wachino J, Kurokawa H, Suzuki S, Yamane K, Shibata N, et al. Emergence of penicillin-resistant group B streptococci. ICAAC;2006. p. C2–1286.
5. Kim HY, Uh Y. Macrolide resistance in β-hemolytic streptococci: changes after the implementation of the separation of prescribing and dispensing of medications policy in Korea. Yonsei Med J. 2004; 45:591–7.

6. Ruess M, Müller U, Sander A, Berner R. Antimicrobial susceptibility patterns of Streptococcus agalactiae in a German university hospital. Scand J Infect Dis. 2000; 32:623–6.
7. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. CLSI document M100-S18 Wayne, PA; CLSI,. 2008.
8. Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. CLSI document M7-A7 Wayne, PA; CLSI,. 2006.
9. Dahesh S, Hensler ME, Van Sorge NM, Gertz RE Jr, Schrag S, Nizet V, et al. Point mutation in the group B streptococcal pbp2x gene conferring decreased susceptibility to β-lactam antibiotics. Antimicrob Agents Chemother. 2008; 52:2915–8.
10. de Azavedo JCS, McGavin M, Duncan C, Low DE, McGeer A. Prevalence and mechanisms of macrolide resistance in invasive and noninvasive group B streptococcus isolates from Ontario, Canada. Antimicrob Agents Chemother. 2001; 45:3504–8.

11. Yi KS, Yong DE, Cho DH, Lee KW, Kim DS, Chong YS. Trend in the isolation of β-hemolytic streptococci and current infection status of group B streptococcus. Korean J Clin Pathol. 2001; 21:365–70.
12. Simoes JA, Aroutcheva AA, Heimler I, Faro S. Antibiotic resistance patterns of group B streptococcal clinical isolates. Infect Dis Obstet Gynecol. 2004; 12:1–8.

13. Iimura T. Group B streptococcal infection. Modern Media. 1979; 25:505–19.
15. Betriu C, Gomez M, Sanchez A, Cruceyra A, Romero J, Picazo JJ. Antibiotic resistance and penicillin tolerance in clinical isolates of group B streptococci. Antimicrob Agents Chemother. 1994; 38:2183–6.

16. Morikawa Y, Kitazato M, Katsukawa C, Tamaru A. Prevalence of cefotaxime resistance in group B streptococcus isolates from Osaka, Japan. J Infect Chemother. 2003; 9:131–3.

17. Uh Y, Hwang GY, Jang IH, Cho HM, Noh SM, Kim HY, et al. Macrolide resistance trends in β-hemolytic streptococci in a tertiary Korean hospital. Yonsei Med J. 2007; 48:773–8.

18. Desjardins M, Delgaty KL, Ramotar K, Seetaram C, Toye B. Prevalence and mechanisms of erythromycin resistance in group A and group B streptococcus: implications for reporting susceptibility results. J Clin Microbiol. 2004; 42:5620–3.
Fig. 1.
MLS genotype analysis of erythromycin-resistant GBS strains. Lane M: 100 bp DNA ladder marker; Lane 1,2,4,5: Negative patient's strains; Lane 3,6,7,10: ermB gene positive strains; Lane 8: Negative control; Lane 9: ermA/TR gene positive strains; Lane 11: mefA/E gene positive strains; Lane 12: Positive control.
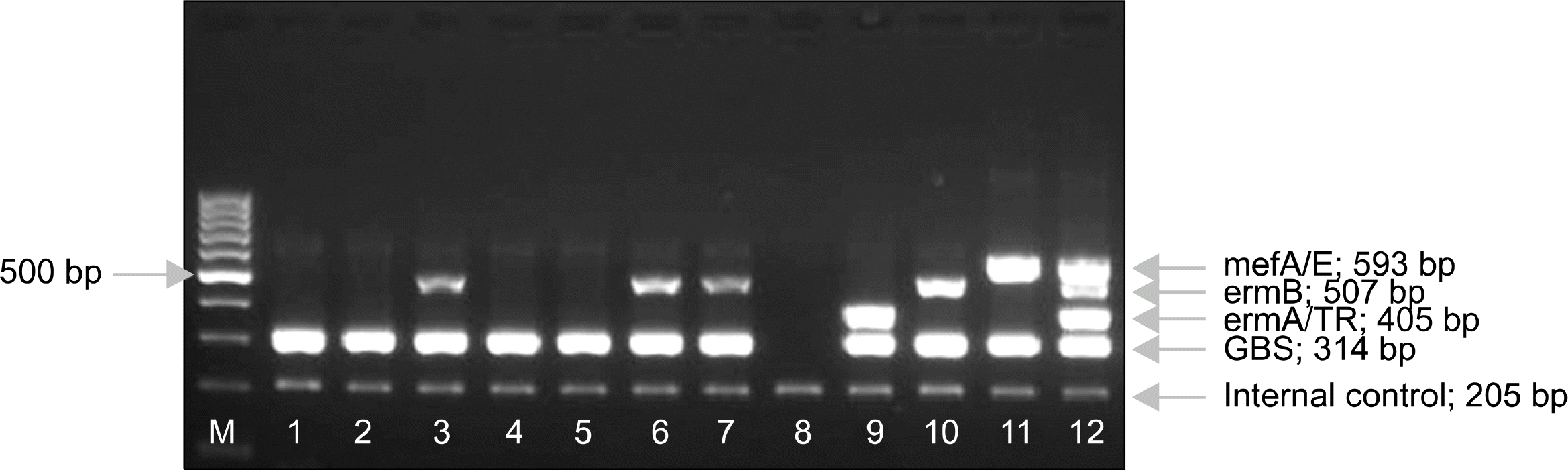
Table 1.
Disc diffusion susceptibility test results of 180 GBS strain
| Antibiotics | % susceptible |
|---|---|
| Penicillin | 180/180 (100) |
| Ampicillin | 180/180 (100) |
| Clindamycin | 102/180 (58.9) |
| Erythromycin | 130/180 (72.2) |
| Cefazolin | 180/180 (100) |
| Vancomycin | 180/180 (100) |
| D-test | 3∗/6 |
Table 2.
Microbroth dilution antimicrobial susceptibility test results of 180 GBS strains
| Antibiotics | MIC (μg/mL)50/90∗ | MIC range |
|---|---|---|
| Penicillin | ≤0.06/≤0.06 | ≤0.06 |
| Oxacillin | ≤0.5/≤0.5 | ≤0.5 |
| Cefotaxime | ≤0.06/≤0.06 | ≤0.06 |
| Cefoxitin | 4/4 | 2∼4 |
Table 3.
MicroScan MIC susceptibility test of 47 erythromycin nonsusceptible GBS strains∗




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download