Abstract
Since vancomycin-resistant enterococci (VRE) were first isolated in Europe, rates of VRE colonization and infection have risen steadily. Today VRE have emerged as important nosocomial pathogens worldwide; hence, it is crucial to understand the underlying mechanism in the spreading of VRE. This article reviews the mechanism of resistance to vanco-mycin and global epidemiology of VRE, as well as the current molecular techniques that are being ap-plied to the epidemiological studies of VRE.
Go to : 
References
1. Leclercq R, Derlot E, Duval J, Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med. 1988; 319:157–61.

2. NNIS (2004) National Nosocomial Infections Surveillance (NNIS) System Report, Data Summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004; 32:470–85.
3. EARSS. EARSS web sites. EARSS Annual Report. 2006. http://www.rivm.nl/earss/. [Online] (last visited on 26 June 2008).
4. Kim JM and Song YG. Vancomycin-resistant enterococcal infections in Korea. Yonsei Med J. 1998; 39:562–8.

5. Shin JW, Yong D, Kim MS, Chang KH, Lee K, Kim JM, et al. Sudden increase of vancomycin-resistant enterococcal infections in a Korean tertiary care hospital: possible consequences of increased use of oral vancomycin. J Infect Chemother. 2003; 9:62–7.

6. Werner G, Klare I, Heier H, Hinz KH, Bohme G, Wendt M, et al. Quinupristin/dalfopristin-resistant enterococci of the satA (vatD) and satG (vatE) genotypes from different ecological origins in Germany. Microb Drug Resist. 2000; 6:37–47.
7. Baysallar M, Kilic A, Aydogan H, Cilli F, Doganci L. Linezolid and quinupristin/dalfopristin resistance in vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus prior to clinical use in Turkey. Int J Antimicrob Agents. 2004; 23:510–2.

8. Herrero IA, Issa NC, Patel R. Nosocomial spread of linezolid-resistant, vancomycin-resistant Enterococcus faecium. New Engl J Med. 2002; 346:867–9.
9. Bae HG, Sung H, Kim MN, Lee EJ, Koo LS. First report of a linezolid-and vancomycin-resistant Enterococcus faecium strain in Korea. Scand J Infect Dis. 2006; 38:383–6.
10. Centers for Disease Control and Prevention. Staphylococcus aureus resistant to vancomycin-United States, 2002. Morb Mortal Wky Rep. 2002; 51:565–7.
11. Centers for Disease Control and Prevention. Vancomycin-resistant Staphylococcus aureus-New York, 2004. Morb Mortal Wky Rep. 2004; 53:322–3.
12. Clark NC, Weigel LM, Patel JB, Tenover FC. Comparison of Tn1546-like elements in vancomycin-resistant Staphylococcus aureus isolates from Michigan and Pennsylvania. Antimicrob Agents Chemother. 2005; 49:470–2.
13. Zhu W, Clark NC, McDougal LK, Hageman J, McDonald LC, Patel JB. vancomycin-resistant Staphylococcus aureus isolates associated with Inc18-like vanA plasmids in Michigan. Antimicrob Agents Chemother. 2008; 52:452–7.
15. Uttley AH, Collins CH, Naidoo J, George RC. Vancomycin-resistant enterococci. Lancet. 1988; 1:57–8.

17. Arthur M and Couvalin P. Genetics and mechanisms of glycopeptide resistance in enterococci. Antimicrob Agents Chemother. 1993; 37:1563–71.

18. Reynolds PE and Courvalin P. Vancomycin resistance due to synthesis of precursors terminating in D-alanyl-D-alanine. Antimicrob Agents Chemother. 2005; 49:21–5.
19. Arthur M, Molinas C, Depardieu F, Courvalin P. Characterization of Tn1546, a Tn3-related transposon conferring glycopeptide resistance by synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J Bacteriol. 1993; 175:117–27.

20. Brown AR, Townsley AC, Amyes SGB. Diversity of Tn1546 elements in clinical isolates of glycopeptide-resistant enterococci from Scottish hospitals. Antimicrob Agents Chemother. 2001; 45:1309–11.
21. Handwerger S, Skoble J, Discotto LF, Pucci MJ. Heterogeneity of the vanA gene in clinical isolates of enterococci from the Northeastern United States. Antimicrob Agents Chemother. 1995; 39:362–8.
22. Darini ALC, Palepou MI, Woodford N. Nucleotide sequence of IS1542, an insertion sequence identified within VanA glycopeptide resistance elements of enterococci. FEMS Microbiol Lett. 1999; 173:341–6.
23. Simonsen GS, Myhre MR, Dahl KH, Olsvik O, Sundsfjord A. Typeability of Tn1546-like elements in vancomycin-resistant enterococci using Long-range PCRs and specific analysis of polymorphic regions. Microbiol Drug Resist. 2000; 6:49–57.

24. Jensen LB, Ahrens P, Dons L, Jones RN, Hammerum AM, Aarestrup FM. Molecular analysis of Tn1546 in Enterococcus faecium isolated from animals & humans. J Clin Microbiol. 1998; 36:437–42.
25. Hashimoto Y, Tanimoto K, Ozawa Y, Murata T, Ike Y. Amino acid substitutions in the vanS sensor of the vanA type vancomycin resistant enterococcus strains result in high-level vancomycin resistance and low-level teicoplanin resistance. FEMS Microbiology Lett. 2000; 185:247–54.
26. Lauderdale TL, McDonald LC, Shiau YR, Chen PC, Wang HY, Lai JF, et al. Vancomycin-resistant enterococci from humans and retail chickens in Taiwan with unique VanB phenotype-vanA genotype incongruence. Antimicrob Agents Chemother. 2002; 46:525–7.
27. Lee WG, Huh JY, Cho SR, Lim YA. Reduction in glycopeptide resistance in vancomycin-resistant enterococci as a result of vanA cluster rearrangements. Antimicrob Agents Chemother. 2004; 48:1379–81.
28. Arthur M, Molinas C, Courvallin P. The VanS VanR two-component regulatory system controls synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J Bacteriol. 1992; 174:2582–91.
29. Song JH, Ko KS, Suh JY, Oh WS, Kang CI, Chung DR, et al. Clinical implications of vancomycin-resistant Enterococcus faecium (VRE) with VanD phenotype and vanA genotype. J Antimicrob Chenother. 2008; 61:838–44.

30. Quintiliani RJ and Courvalin P. Characterization of Tn1547, a composite transposon flanked by the IS16 and IS256-like elements, that confers vancomycin resistance in Enterococcus faecalis BM4281. Gene. 1996; 172:1–8.

31. Carias LL, Rudin SD, Donskey CJ, Rice LB. Genetic linkage and cotransfer of a novel, vanB-containing transposon (Tn5382) and a low-affinity penicillin-binding protein 5 gene in a clinical vancomycin-resistant Enterococcus faecium isolate. J Bactriol. 1998; 180:4426–34.
32. Dahl KH, Simonsen GS, Olsvik O, Sundsfjord A. Heterogeneity in the vanB gene cluster of genomically diverse clinical strains of vancomycin-resistant enterococci. Antimicrob Agents Chemother. 1999; 43:1105–10.
33. Lee WG, Jernigan JA, Rasheed JK, Anderson GJ, Tenover FC. Possible horizontal transfer of the vanB2 gene among genetically diverse strains of vancomycin-resistant Enterococcus faecium in a Korean hospital. J Clin Microbiol. 2001; 39:1165–8.
34. Dutka-Malen S, Molinas C, Arthur M, Cournalin P. Sequence of the vanC gene of Enterococcus gallinarum BM4174 encoding a D-alanine: D-alanine ligase-related protein necessary for vancomycin resistance. Gene. 1992; 112:53–8.
35. Toye B, Shymanski J, Bobrowska M, Woods W, Ramotar K. Clinical and epidemiological significance of enterococci intrinsically resistant to vancomycin (possessing the vanC genotype). J Clin Microbiol. 1997; 35:3166–70.

36. Corso A, Faccone D, Gagetti P, Togneri A, Lopardo H, Melano R, et al. First report of VanA Enterococcus gallinarum dissemination within an intensive care unit in Argentina. Int J Antimicrob Agents. 2005; 25:51–6.

37. Lee H, Koh EM, Kim MS, Yum JH, Yong D, Lee WG, et al. Comparison of Tn1546-like elements in vanA genotype Enterococcus gallinarum and E. faecalis or E. faecium. Korean J Clin Microbiol. 2007; 10:114–8.
38. Perichon B, Reynolds P, Courvalin P. VanD-type glycopeptide-resistant Entercococcus faecium BM4339. Antimicrob Agents Chemother. 1997; 41:2016–8.
39. Camargo IL, Dalla Costa LM, Woodford N, Gilmore MS, Darini AL. Sequence analysis of Enterococcus faecium strain 10/96A (VanD4), the original vancomycin-resistant E. faecium strain in Brazil. J Clin Microb. 2006; 44:2635–7.
40. Boyd DA, Kibsey P, Roscoe D, Mulvey MR. Enterococcus faecium N03–0072 carries a new VanD-type vancomycin resistance determinant: characterization of the VanD5 operon. J Antimicrob Chemother. 2004; 54:680–3.

41. Fines M, Perchon B, Reynolds P. VanE, a new type of acquired glycopeptide resistance in Enterococcus faecalis BM4405. Antimicrob Agents Chemother. 1999; 43:2161–4.
42. McKessar SJ, Berry AM, Bell JM. Genetic characterization of vanG, a novel vancomycin resistance locus in Enterococcus faecalis. Antimicrob Agents Chemother. 2000; 44:3224–8.
43. Van Bambeke F, Chauvel M, Reynolds PE, Fraimow HS, Courvalin P. Vancomycin-dependent Enterococcus faecalis clinical isolates and revertant mutants. Antimicrob Agents Chemother. 1999; 43:41–7.
44. Tambyah PA, Marx JA, Maki DG. Nosocomial infection with vancomycin-dependent enterococci. Emerg Infect Dis. 2004; 10:1277–81.
45. Stobberingh E, van den Bogaard A, London N, Driessen C, Top J, Willems R. Enterococci with glycopeptide resistance in turkeys, turkey farmers, turkey slaughterers, and (sub)urban residents in the south of the Netherlands: evidence for transmission of vancomycin resistance from animals to humans? Antimicrob Agents Chemother. 1999; 43:2215–21.

46. Mascini EM and Bonten JM. vancomycin-resistant enterococci: consequences for therapy and infection control. Clin Microbiol Infect. 2005; 11:43–56.
47. Kirst HA, Thompson DG, Nicas TI. Historical yearly usage of vancomycin. Antimicrob Agents Chemother. 1998; 42:1303–4.

48. Donskey CJ, Schreiber JR, Jacobs MR, Shekar R, Salata RA, Gordon S, et al. A polyclonal outbreak of predominantly vanB vancomycin-resistant enterococci in northeast Ohio. Northeast Ohio vancomycin-resistant Enterococcus Surveillance Program. Clin Infect Dis. 1999; 29:573–9.
49. Kim WJ, Weinstein RA, Hayden MK. The changing molecular epidemiology and establishment of endemicity of vancomycin resistance in enterococci at one hospital over a 6-year period. J Infect Dis. 1999; 179:163–71.

50. Lee H, Yong D, Kim MS, Yum JH, Lee WG, Huh JY, et al. Antimicrobial susceptibilities and PFGE patterns of vancomycin-resistant Enterococcus isolated from clinical specimens and chickens. Korean J Lab Med. 2005; 25:39–45.
51. Jung WK, Hong SK, Lim JY, Lim SK, Kwon NH, Kim JM, et al. Phenotypic and genetic characterization of vancomycin-resistant enterococci from hospitalized humans and from poultry in Korea. FEMS Microbiol Lett. 2006; 260:193–200.

52. Roghmann MC, Qaiyumi S, Schwalbe R, Morris JG Jr. Natural history of colonization with vancomycin-resistant Enterococcus faecium. Infect Control Hosp Epidemiol. 1997; 18:679–80.

53. HIPAC. Recommendations for preventing the spread of vancomycin resistance. Infect Control Hosp Epidemiol. 1995; 16:105–13.
54. Huh JY, Lee WG, Lee K, Shin WS, Yoo JH. Distribution of insertion sequences associated with Tn1546-like elements among Enterococcus faecium isolates from patients in Korea. J Clin Microbiol. 2004; 42:1897–902.
55. Homan WL, Tribe D, Poznanski S, Li M, Hogg G, Spalburg E, et al. Multilocus sequence typing scheme for Enterococcus faecium. J Clin Microbiol. 2002; 40:1963–71.
56. Leavis HL, Bonten M, Willems RJL. Identification of high-risk enterococcal clonal complexes global dispersion and antibiotic resistance. Curr Opin Microbiol. 2006; 9:454–60.

57. Ko KS, Baek JY, Lee JY, Oh WS, Peck KR, Lee NY, et al. Moleular characterization of vancomycin-resistant Enterococcus faecium isolates from Korea. J Clin Microbiol. 2005; 43:2303–6.
58. Lee WG, Lee SM, Kim YS. Moleular characterization of Enterococcus faecium isolated from hospitalized patients in Korea. Lett Appl Microbiol. 2006; 43:274–9.
59. Peta M, Carretto E, Barbarini D, Zamperoni A, Carnevale L, Perversi L, et al. Outbreak of vancomycin-resistant Enterococcus spp. in an Italian general intensive care unit. Clin Microbiol Infect. 2006; 12:163–9.

60. Klare I, Konstabel C, Mueller-Bertling S, Werner G, Strommenger B, Kettlitz C, et al. Spread of ampicillin/vancomycin-resistant Enterococcus faecium of the epidemic-virulent clonal complex-17 carrying the genes esp and hyl in German hospitals. Eur J Clin Microbiol Infect Dis. 2005; 24:815–25.

61. Top J, Schouls LM, Bonten M, Willems RJ. Multiple-locus variable-number tandem repeat analysis, a novel typing scheme to study the genetic relatedness and epidemiology of Enterococcus faecium isolates. J Clin Microbiol. 2004; 42:4503–11.
62. Willems RJ, Top J, van Santen M, Robinson DA, Coque TM, Baquero F, et al. Global spread of vancomycin-resistant Enterococcus faecium from distinct nosocomial genetic complex. Emerg Infect Dis. 2005; 11:821–8.
63. Top J, Willems RJ, van der Velden S, Asbroek M, Bonten M. Emergence of clonal complex 17 Enterococcus faecium in the Netherlands. J Clin Microbiol. 2008; 46:214–9.
Go to : 
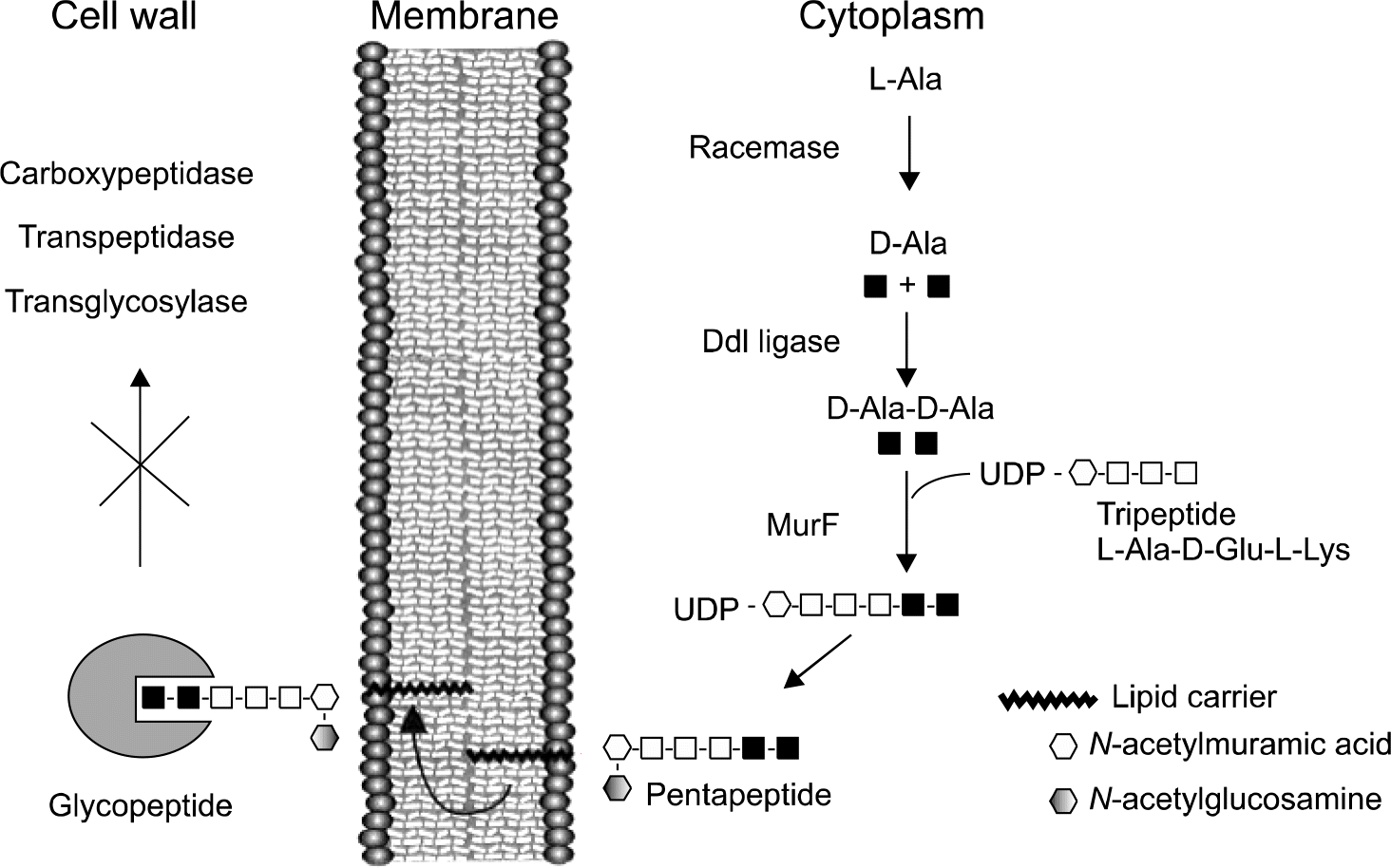 | Fig. 1.Peptidoglycan biosynthesis and mechanism of action of vancomycin. Binding of the antibiotic to the C-terminal D-Ala-D-Ala of late peptidoglycan precursors prevents reactions catalyzed by trans-glycosylases, transpeptidases, and D,D-carboxypeptidases. Ddl, D-Ala: D-Ala ligase; MurF, a synthetase protein; UDP, uracil diphosphate. Adapted from reference 16. |
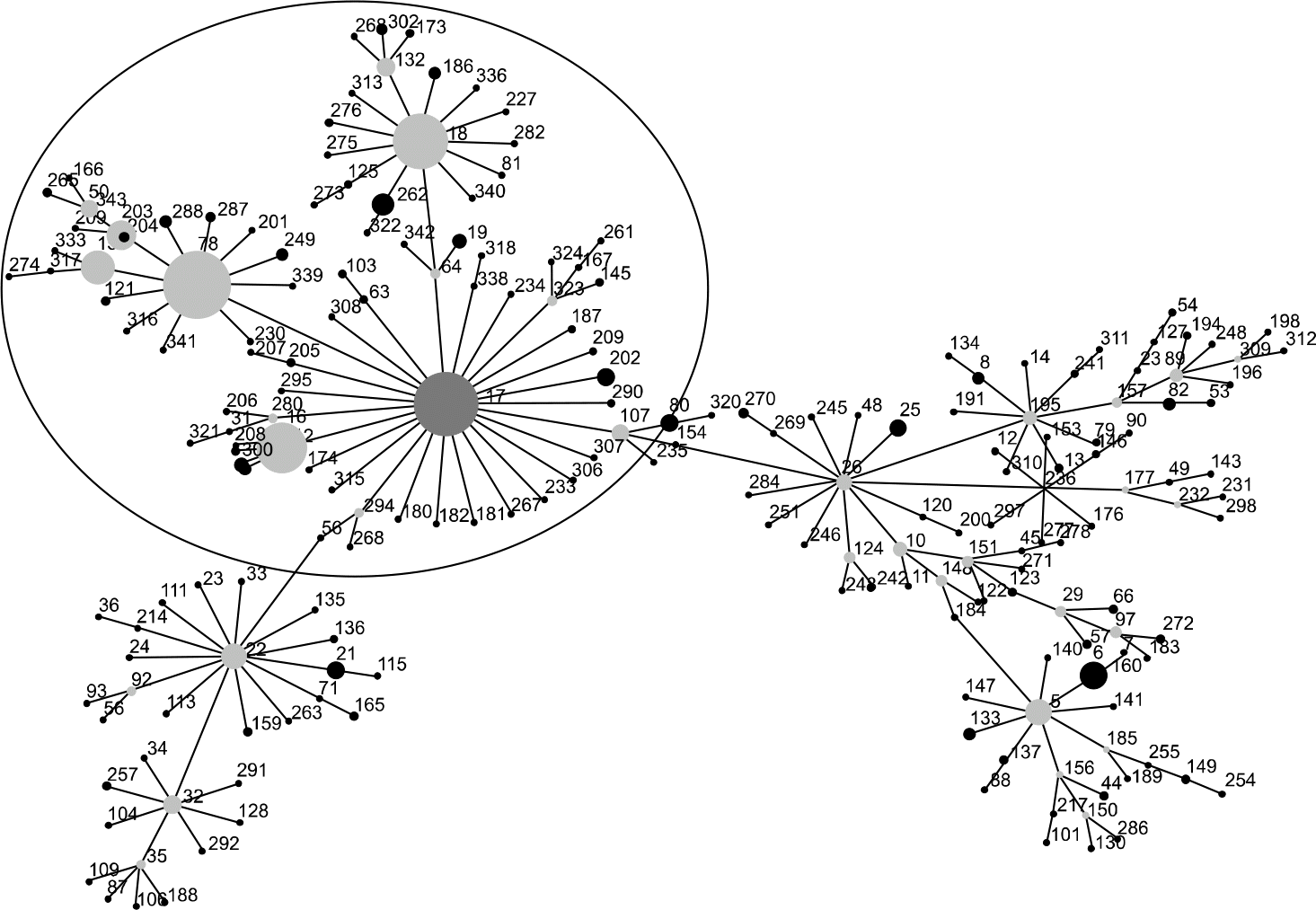 | Fig. 2.eBURST diagram of the analysis of the sequence types (STs) of the entire public E. faecium MLST database. The circle indicates the CC17. Adapted from MLST web sites. http://www.mlst.net (last visited on 26 June 2008). |
Table 1.
Level and type of resistance to vancomycin in enterococci. Adapted from reference 16




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download