Abstract
Background
We noticed a sudden increase in the isolation of Klebsiella oxytoca from bronchial washing specimens during May to June 2006. An epidemiological investigation was conducted to identify the cause of the outbreak and to implement appropriate infection control measures.
Methods
A total of 18 isolates of K. oxytoca were found. The 14 bronchial washing specimens that yielded K. oxytoca were taken in the outpatient bronchoscopy suite, and the other 4 specimens were obtained by a portable bronchoscopy. The medical records and microbiologic findings of these patients were reviewed. Environmental samples from two bron-choscopes and the bronchoscopy suite were cultured. The relations between the available 10 isolates from bronchial washing fluid were investigated by pulsed-field gel electrophoresis (PFGE).
Results
No patients were judged to have had true infections attributable to K. oxytoca either before or after bronchoscopy. Cultures of samples from two bronchoscopes and related environment did not grow K. oxytoca. The PFGE analysis showed that 8 of 10 isolates had a similar pattern of DNA fragments. An infection control strategy was implemented, including adequately cleaning and disinfecting the broncho-scopes, and a sharp reduction in the incidence of K. oxytoca from bronchial washing samples followed.
References
1. Podschun R and Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998; 11:589–603.
2. French G and Philips I. Antimicrobial resistance in hospital flora and nosocomial infection; Hospital Epidemiology and Infection. In:. Mayhall CG, editor. Hospital epidemiology and infection control. 2nd ed.Philadelphia: Lippincott Williams & Wilkins;2006. p. 1243–64.
3. Decre D, Burghoffer B, Gautier V, Petit JC, Arlet G. Outbreak of multi-resistant Klebsiella oxytoca involving strains with extended-spectrum beta-lactamases and strains with extended-spectrum activity of the chromosomal beta-lactamase. J Antimicrob Chemother. 2004; 54:881–8.
4. Watson JT, Jones RC, Siston AM, Fernandez JR, Martin K, Beck E, et al. Outbreak of catheter-associated Klebsiella oxytoca and Enterobacter cloacae bloodstream infections in an oncology chemotherapy center. Arch Intern Med. 2005; 165:2639–43.

5. Berthelot P, Grattard F, Patural H, Ros A, Jelassi-Saoudin H, Pozzetto B, et al. Nosocomial colonization of premature babies with Klebsiella oxytoca: probable role of enteral feeding procedure in transmission and control of the outbreak with the use of gloves. Infect Control Hosp Epidemiol. 2001; 22:148–51.

6. Reiss I, Borkhardt A, Fussle R, Sziegoleit A, Gortner L. Disinfectant contaminated with Klebsiella oxytoca as a source of sepsis in babies. Lancet. 2000; 356:310.

7. Sardan YC, Zarakolu P, Altun B, Yildirim A, Yildirim G, Hascelik G, et al. A cluster of nosocomial Klebsiella oxytoca bloodstream infections in a university hospital. Infect Control Hosp Epidemiol. 2004; 25:878–82.

8. Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988; 16:128–40.

9. Diekema DJ and Pfaller MA. Infection control epidemiology and clinical microbiology. Murray PR, editor. Manual of clinical microbiology. 8th ed.Washington, D.C: ASM Press;2006. p. 118–28.
10. Bou R, Aguilar A, Perpinan J, Ramos P, Peris M, Lorente L, et al. Nosocomial outbreak of Pseudomonas aeruginosa infections related to a flexible bronchoscope. J Hosp Infect. 2006; 64:129–35.

11. Corne P, Godreuil S, Jean-Pierre H, Jonquet O, Campos J, Jumas-Bilak E, et al. Unusual implication of biopsy forceps in outbreaks of Pseudomonas aeruginosa infections and pseudo-infections related to bronchoscopy. J Hosp Infect. 2005; 61:20–6.

12. Srinivasan A, Wolfenden LL, Song X, Mackie K, Hartsell TL, Jones HD, et al. An outbreak of Pseudomonas aeruginosa infections associated with flexible bronchoscopes. N Engl J Med. 2003; 348:221–7.

13. Silva CV, Magalhaes VD, Pereira CR, Kawagoe JY, Ikura C, Ganc AJ. Pseudo-outbreak of Pseudomonas aeruginosa and Serratia marcescens related to bronchoscopes. Infect Control Hosp Epidemiol. 2003; 24:195–7.
14. Kressel AB and Kidd F. Pseudo-outbreak of Mycobacterium chelonae and Methylobacterium mesophilicum caused by contamination of an automated endoscopy washer. Infect Control Hosp Epidemiol. 2001; 22:414–8.
15. Wilson SJ, Everts RJ, Kirkland KB, Sexton DJ. A pseudo-outbreak of Aureobasidium species lower respiratory tract infections caused by reuse of single-use stopcocks during bronchoscopy. Infect Control Hosp Epidemiol. 2000; 21:470–2.
16. Ahn GY, Yu FN, Jang SJ, Kim DM, Park G, Moon DS, et al. Pseudo-outbreak of Stenotrophomonas maltophilia Due to Contamination of Bronchoscope. Korean J Lab Med. 2007; 27:205–9.

17. Koh EH, Kim S, Bae IG. Epidemiologic investigation of an outbreak of Serratia marcescens urinary tract infection in an intensive care unit using pulsed-field gel electrophoresis. Korean J Clin Microbiol. 2005; 8:34–40.
Fig. 2.
Epidemic curve of K. oxytoca isolates from bronchial washing samples collected during May and June. Solid bars, number of isolates related to the outbreak determined by PFGE analysis; striped bars, number of isolates unrelated to the outbreak determined by PFGE; open bars, number of isolates unavailable for PFGE.
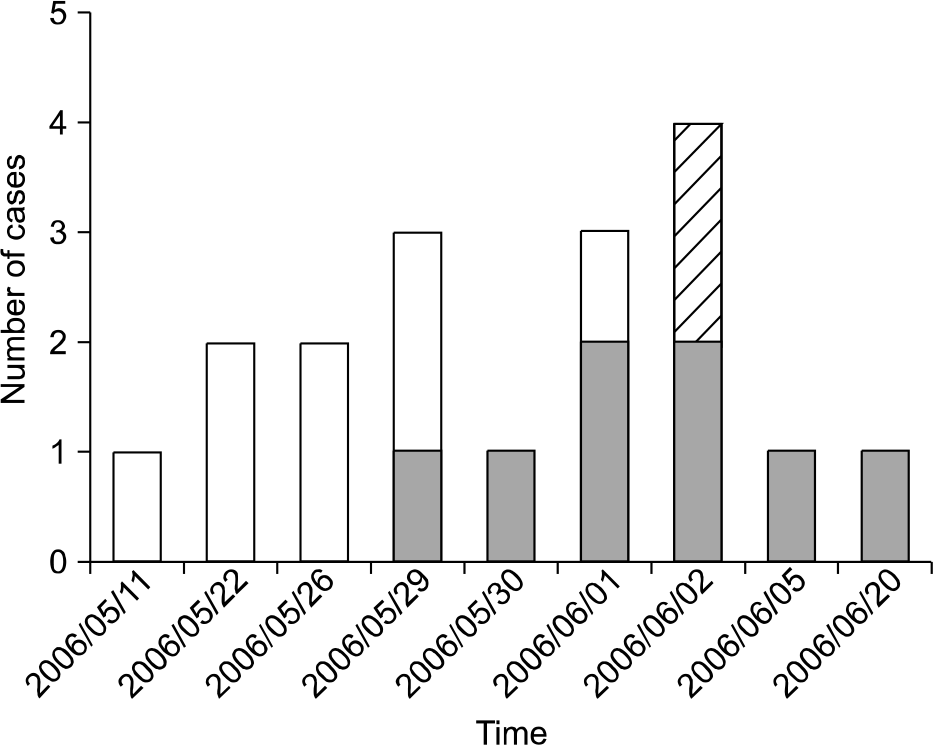
Table 1.
Patient characteristics and the dates of the bronchial washing cultures that grew K. oxytoca




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download