Abstract
Background
Since the emergence of variant Clostridium difficile strains that fail to produce detectable toxin A, diagnostic kits targeted to detect toxin A only showed a considerable rate of false negative results. The aim of this study was to evaluate a toxins A and B (toxins A/B) detection kit recently marketed in Korea, and to compare toxin positive rates before and after introduction of the new kit.
Methods
The results of 5,783 toxin A assays performed during the 7-year period from 2001 through 2007 were analyzed and compared them to the toxins A/B assay data of 519 samples obtained from January to June 2008 in a university hospital. An enzyme-linked fluorescent immunoassay for toxins A/B (VIDAS C. difficile Toxin A & B, bioMerieux SA, France: VIDAS CDAB) and PCR for toxin genes A/B were performed directly in 102 stool samples from hospitalized patients.
Results
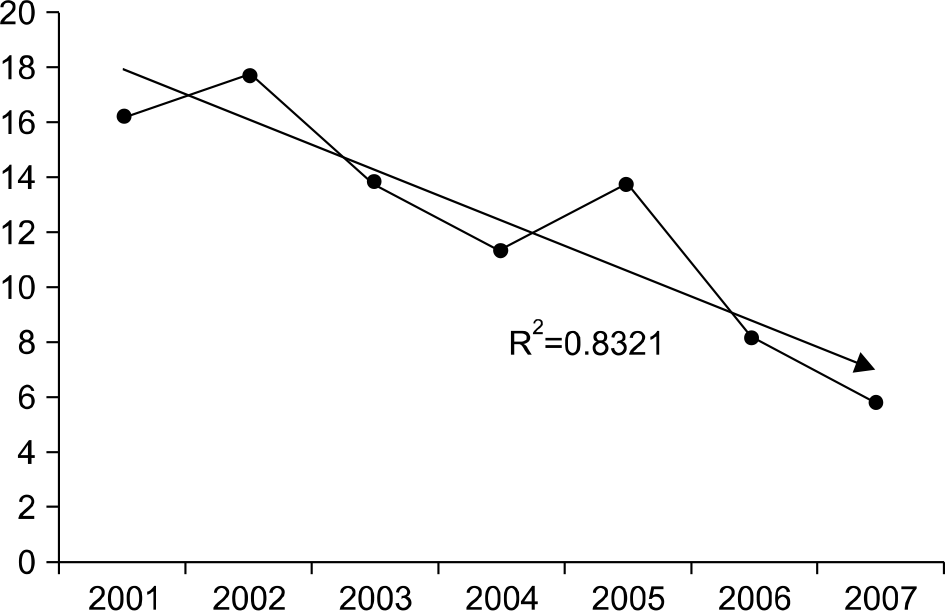
The positive rates of toxin A assays tended downward annually from 2001 to 2007 (16.3%, 17.8%, 13.9%, 11.4%, 13.8%, 8.2%, and 5.8%, respectively), but increased to 12.1% in 2008 after changing to the toxin A/B detection kit. The concordant rate of the VIDAS CDAB kit with the PCR method was 82.4%. Compared to the PCR method, the sensitivity and specificity of the toxin A/B kit were 60.7% and 90.5% respectively.
Go to : 
References
1. Alfa MJ, Kabani A, Lyerly D, Moncrief S, Neville LM, Al-Barrak A, et al. Characterization of a toxin A-negative, toxin B-positive strain of Clostridium difficile responsible for a nosocomial outbreak of Clostridium difficile-associated diarrhea. J Clin Microbiol. 2000; 38:2706–14.
2. Drudy D, Fanning S, Kyne L. Toxin A-negative, toxin B-positive Clostridium difficile. Int J Infect Dis. 2007; 11:5–10.

3. Shin BM and Kuak EY. Characterization of a toxin A negative and toxin B positive variant strain of Clostridium difficile. Korean J Lab Med. 2006; 26:27–31.
4. Rupnik M. Heterogeneity of large clostridial toxins: importance of Clostridium difficile toxinotypes. FEMS Microbiol Rev. 2008; 32:541–55.
5. Shin BM and Lee EJ. Comparison of toxin A enzyme linked fluorescence assay and latex agglutination based on Clostridium difficile culture and toxin A and B PCR assay. Korean J Clin Microbiol. 2005; 8:130–5.
6. Drudy D, Harnedy N, Fanning S, O'Mahony R, Kyne L. Isolation and characterisation of toxin A-negative, toxin B-positive Clostridium difficile in Dublin, Ireland. Clin Microbiol Infect. 2007; 13:298–304.

7. Reyes RC, John MA, Ayotte DL, Covacich A, Milburn S, Hussain Z. Performance of TechLab C. DIFF QUIK CHEKTM and TechLab C. DIFFICILE TOX A/B IITM for the detection of Clostridium difficile in stool samples. Diagn Microbiol Infect Dis. 2007; 59:33–7.
8. Kato H, Kato N, Watanabe K, Iwai N, Nakamura H, Yamamoto T, et al. Identification of toxin A negative, toxin B positive Clostridium difficile by PCR. J Clin Microbiol. 1998; 36:2178–82.
9. Landry ML, Topal J, Ferguson D, Giudetti D, Tang Y. Evaluation of biosite triage Clostridium difficile panel for rapid detection of Clostridium difficile in stool samples. J Clin Microbiol. 2001; 39:1855–8.
10. O'Connor D, Hynes P, Cormican M, Collins E, Corbett-Feeney G, Cassidy M. Evaluation of methods for detection of toxins in specimens of feces submitted for diagnosis of Clostridium difficile-associated diarrhea. J Clin Microbiol. 2001; 39:2846–9.
11. Turgeon DK, Novicki TJ, Quick J, Carlson LD, Miller P, Ulness B, et al. Six rapid tests for direct detection of Clostridium difficile and its toxins in fecal samples compared with the fibroblast cytotoxicity assay. J Clin Microbiol. 2003; 41:667–70.
12. Bouza E, Pelaez T, Alonso R, Catalan P, Munoz P, Creixems MR. “Second-look” cytotoxicity: an evaluation of culture plus cytotoxin assay of Clostridium difficile isolates in the laboratory diagnosis of CDAD. J Hosp Infect. 2001; 48:233–7.

13. Lozniewski A, Rabaud C, Dotto E, Weber M, Mory F. Laboratory diagnosis of Clostridium difficile-associated diarrhea and colitis: usefulness of Premier Cytoclone A1B enzyme immunoassay for combined detection of stool toxins and toxigenic C. difficile strains. J Clin Microbiol. 2001; 39:1996–8.
14. Lipson SM, Tortora G, Tempone A, Fedorko DP, Spitzer ED. Rapid detection of Clostridium difficile in stool using the VIDASR C. difficile Toxin A II assay. Diagn Microbiol Infect Dis. 2003; 45:117–21.

15. Snell H, Ramos M, Longo S, John M, Hussain Z. Performance of the TechLab C. DIFF CHEK-60 enzyme immunoassay (EIA) In combination with the C. difficile Tox A/B II EIA kit, the Triage C. difficile panel immunoassay, and a cytotoxin assay for diagnosis of Clostridium difficile-associated diarrhea. J Clin Microbiol. 2004; 42:4863–5.
16. Yoo SJ, Kang JO, Oh HJ, Shin BM. Comparison of two enzyme immunoassays for Clostridium difficile toxin A. Korean J Lab Med. 2006; 26:408–11.

17. Rüssmann H, Panthel K, Bader RC, Schmitt C, Schaumann R. Evaluation of three rapid assays for detection of Clostridium difficile toxin A and toxin B in stool specimens. Eur J Clin Microbiol Infect Dis. 2007; 26:115–9.

18. Borriello SP, Wren BW, Hyde S, Seddon SV, Sibbons P, Krishna MM, et al. Molecular, immunological, and biological characterization of a toxin A negative, toxin B-positive strain of Clostridium difficile. Infect Immun. 1992; 60:4192–9.
19. Lyerly DM, Barroso LA, Wilkins TD, Depitre C, Corthier G. Characterization of a toxin A-negative, toxin B-positive strain of Clostridium difficile. Infect Immun. 1992; 60:4633–9.

20. Drudy D, Harnedy N, Fanning S, O'Mahony R, Kyne L. Isolation and characterisation of toxin A-negative, toxin B positive Clostridium difficile in Dublin, Ireland. Clin Microbiol Infect. 2007; 13:298–304.
21. Kang JO, Chae JD, Eom JI, Han D, Park PW, Park IK, et al. Comparison of Clostridium difficile toxin A immunoassay with cytotoxicity assay. Korean J Clin Microbiol. 2000; 3:43–7.
22. Kim H, Riley TV, Kim M, Kim CK, Yong D, Lee K, et al. Increasing prevalence of toxin A-negative, toxin B-positive isolates of Clostridium difficile in Korea: impact on laboratory diagnosis. J Clin Microbiol. 2008; 46:1116–7.
23. Delmee M, Van Broeck J, Simon A, Janssens M, Avesani V. Laboratory diagnosis of Clostridium difficile-associated diarrhoea: a plea for culture. J Med Microbiol. 2005; 54:187–91.
24. Shin BM, Kuak EY, Yoo HM, Kim EC, Kang JO, Whang DH, et al. Multicentre study of the prevalence of toxigenic Clostridium difficile in Korea: results of a retrospective stydy 2000–2005. J Med Microbiol. 2008; 57:697–701.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download