Abstract
Biodegradable implants made of co-polymers composed of L-lactide, D-lactide, and trimethylene carbonate were used in the present case. To our knowledge, only one reported tissue reaction has been associated with ankle fracture treated with third-generation implants internationally and none yet domestically. We report a delayed foreign-body reaction of ankle fracture treated with a third-generation biodegradable plate and screws. We suggest that ankle fracture patients treated with biodegradable implants should be advised of this possible complication and should be followed for at least 2 years.
First-generation bioabsorbable implants consisting mainly of polyglycolic acid appeared in the 1990s. They displayed rapid degradation and high rates of subsequent inflammatory reactions1). Second-generation implants consisted of poly-L-lactide and showed substantial improvements in degradation rates and foreign-body reactions1). Third-generation implants composed of trimethylene carbonate, L-lactide, and D, L-lactide displayed improved strength and slow degradation rates (2~4 years)5~7). These were designed to overcome the problems of rapidly diminishing strength and frequent foreign-body reactions5~7). However, the prolonged degradation rate of new implants has raised another concerns. One of the major concerns is whether these materials actually diminish adverse soft tissue reactions or the reactions simply are postponed to a later time. These bioabsorbable implants have been studied extensively in vitro and in animal models5,7), but only few short-term studies were conducted in humans4,9). To our knowledge, only one reported tissue reaction have been associated with ankle fracture treated with Third-generation implants internationally3) and not yet domestically. We report delayed foreign-body reaction of ankle fracture treated with Third-generation biodegradable plate and screws.
A 21-year-old male soldier sustained a closed, left lateral malleolar fracture when he slipped during soccer game in March 2010. He had no known systemic diseases and reported no allergy. One week after the injury, he underwent open reduction and internal fixation with a biodegradable plate and screws (Inion OTPS™, Inion Oy, Tampere, Finland) by other orthopedic surgeon (Fig. 1). The postoperative course was unremarkable. In Febuary 2011, eleven months after the procedure, the patient noted a gradually enlarging soft-tissue mass adjacent to the previous surgical scar (Fig. 2). The mass was soft, nonmovable, and elastic in its character. When pressure was applied, there was mild discomfort but painless. On radiographs, fracture healed but osteolytic change was occurred and magnetic resonance imaging showed a healed lateral malleolar fracture accompanied by oval mass with an accumulation of fluid to sinus formation (Fig. 3). Laboratory data (C-reactive protein level, erythrocyte sedimentation rate and white blood-cell count) were within normal limited.
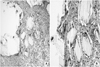
Operative exploration was carried out in March 2011, one year after the initial operation. Intraoperatively, fluid accompanied by several fragments of whitish material was drained. Surrounding granulomatous tissue and all implant remnants were removed, and samples were collected for microbiological and histopathological evaluation (Fig. 4). Intraoperative Gram staining and cultures were negative. Later histopathological analysis revealed a nonspecific foreign-body reaction: uniform histopathologic picture of nonspecific inflammation and abundant polymeric particles surrounded by mononuclear phagocytes and multinucleated foreign-body giant cells (Fig. 5). There was no evidence of infection or malignancy. The surgical wound healing was uneventful. The patient reported a direct clinical improvement, and symptoms subsided as early as 2 weeks postoperatively. He noted no complication between the reoperation and the final follow up (3 months after reoperation).
All biodegradable implants induce a subclinical but histopathologically recognizable non-specific foreign body type of tissue response8). According to Rokkanen et al8), this seems to be a phenomenon inherent in the degradation and absorption processes of these polymers in the tissues. This can be regarded normal as long as it does not cause any clinical symptoms. The process of biodegradation of a polymer implant begins with the polymer chains being broken into smaller fragments by hydrolysis. The molecular weight of the implant decreases first. Thereafter, the mechanical strength of the implant decreases, allowing subsequent mechanical fragmentation and absorption of the implant to begin. Actual mass loss of the implant then occurs through the release of soluble degradation products, phagocytosis by macrophages and histiocytes, intracellular degradation, and finally, metabolic elimination through citric acid (Krebs) cycle to carbon dioxide and water occur, which are expelled from the body via respiration and urine5). Regarding the phagocytic and clearing capacity of the tissues, the most demanding phase is the decomposition stage, when the gross geometry of the implant is rapidly lost. At this time, the production rate of polymeric debris particles may exceed the critical limits of tissue tolerance1). This may occur especially if there is poor vascularity at the implantation site or if there is only a thin soft tissue layer covering the implants1) like when implants are placed on the lateral malleolus. Local accumulation of released monomers may lower the local pH of the tissue1), which may in turn lead to increased osmotic pressure resulting in a temporary local sterile fluid accumulation. The patient notices this reaction as swelling which can be painful if severe. However, such soft tissue reactions do not usually impair bone healing because with the currently used materials they are unlikely to occur during the first 6 months after implantation.
The biodegradable implants used in the present study are made of co-polymers composed of L-lactide, D-lactide and trimethylene carbonate5~7). The plates are 1.2 mm thick and the 2.8~3.1 mm diameters screws have flat low-profile heads. The co-polymer material has been found in sheep to become soft in 6~12 months and to degrade completely by 24 months without any harmful inflammatory or foreign body reactions7). However, in the recent study, subcutaneous implant-degradation related soft tissue reactions were found in four patients (three mild and painless, and one more severe reaction, i.e., 2~8% occurrence rate) 8~18 months postoperatively3). In Korea, we first present a patient with delayed foreign-body reaction 11 months postoperatively. Cho et al2) previously investigated the suitability of the Third-generation biodegradable implants for the treatment of ankle fracture. In their study, 20 patient were treated with a biodegradable plate and screws (Inion OTPS™) and no foreign-body reaction was observed after a mean 5.6-months follow-up. We think the follow-up period of their study was too short.
We suggest modern absorbable implants with slow degradation rates have not eliminated the problem of foreign-body reactions but simply postponed the occurrence of reactions. So ankle fracture patients treated with biodegradable implants should be advised of this possible complication that is foreign-body reaction and should be followed for at least 2 years, possibly longer.
Figures and Tables
Fig. 1
(A) Preoperative radiograph showing a lateral malleolar fracture.
(B) Radiograph made one week after open reduction and internal fixation with biodegradable implants.
(C) Biodegradable plate and screws (Inion OTPS™, Inion Oy, Tampere, Finland).
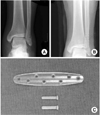
Fig. 2
The patient noted a gradually enlarging soft-tissue mass adjacent to the previous surgical scar.
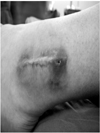
Fig. 3
(A) On radiographs, the fracture had healed but osteolytic change had occurred.
(B) Magnetic resonance imaging showed a healed lateral malleolar fracture accompanied by an oval mass with an accumulation of fluid in the sinus formation.
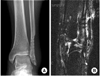
Fig. 4
(A) Intraoperative clinical photographs showing a collection of fluid accompanied by several fragments of whitish material.
(B) Granulomatous tissue and foreign-body fragments.

Fig. 5
(A) Photomicrograph showing some variably shaped, hyaline foreign-body material with a rhomboid shape (H&E stain, ×100).
(B) Photomicrograph showing fragments surrounded by a foreign-body reaction with mononuclear cells, foamy histiocytes, foreign body-type multinucleated giant cells, and granulation tissue in the stroma (H&E stain, ×400).
References
1. Böstman O, Pihlajamäki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials. 2000. 21:2615–2621.

2. Cho JY, Kim JW, Kim SE, Jung KC, Choi SH. Surgical fixation with biodegradable plate for the treatment of ankle fractures. J Korean Fract Soc. 2008. 21:31–36.

3. Kukk A, Nurmi JT. A retrospective follow-up of ankle fracture patients treated with a biodegradable plate and screws. Foot Ankle Surg. 2009. 15:192–197.

4. Laughlin RM, Block MS, Wilk R, Malloy RB, Kent JN. Resorbable plates for the fixation of mandibular fractures: a prospective study. J Oral Maxillofac Surg. 2007. 65:89–96.

5. Losken HW, van Aalst JA, Mooney MP, et al. Biodegradation of Inion fast-absorbing biodegradable plates and screws. J Craniofac Surg. 2008. 19:748–756.

6. Mavrogenis AF, Kanellopoulos AD, Nomikos GN, Papagelopoulos PJ, Soucacos PN. Early experience with biodegradable implants in pediatric patients. Clin Orthop Relat Res. 2009. 467:1591–1598.

7. Nieminen T, Rantala I, Hiidenheimo I, et al. Degradative and mechanical properties of a novel resorbable plating system during a 3-year follow-up in vivo and in vitro. J Mater Sci Mater Med. 2008. 19:1155–1163.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download