Abstract
Purpose
This study investigated the effect of COX-2 inhibitor on the expression of MMP-13 in the healing process of fracture.
Materials and Methods
Adult Sprague-Dawley rats were divided into two groups of twenty five rats each. Unilateral femoral shaft fractures were created artificially under displacement in all two groups. COX-2 inhibitor was only given to the experimental group from the postoperative day 1. At 2 weeks after fracture the rats were sacrificed and the callus from each group was used for histologic examination and real time RT-PCR for MMP-13 expression.
Fracture healing of the bone is a complex process that involves the formation and resorption of the extracellular matrix. The cartilaginous callus is formed at the fracture site and changed to new bone through ossification process. This process is proceeded by rapid replacement of type II collagen with type I collagen, major protein component of bone12).
Matrix metalloproteinases (MMPs) degrade most extracellular matrix protein. Among these enzymes, MMP-13 (collagenase-3) plays prominent role in degradation of type II collagen12). According to this enzymatic activity, MMP-13 is known to be involved in fracture healing process, although it'S mechanism has not been fully clarified7,19).
Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly prescribed as analgesics for acute traumatic and postoperative pain. Because Cyclooxygenase-2 (COX-2) specific inhibitors are less likely to cause gastric ulcer, they are regarding as safer drugs for clinical application than other NASIDs, despite their unclarified role in human bone metabolism3,14). As COX-2 plays an important role in inflammation phase of fracture healing process3,18), selective COX-2 inhibitors have also demonstrated inhibitory effect on fracture healing. Although there are studies that selective COX-2 inhibitor does not have a significant long-term effect on bone healing1,4,9), many studies have shown that COX-2 inhibitors have an inhibitory effects on bone healing, especially in early phase2,5,8,10,14,17). These controversies may be attributed to their transient effect on COX-2 inhibition, which is also related to dosage and duration of therapy1).
The purpose of this study is to investigate the effects of COX-2 inhibitor on expression of MMP-13 in early experimental fracture healing process.
Adult Sprague-Dawley rats weighing average 300 g were divided into two groups of twenty five rats each. Minimally displaced unilateral femoral shaft fractures were produced in all two groups according to the method described by Bonnarens and Einhorn3). One group received no drug as control group, the other group received celebrex® (Pfizer, USA) at a dose of 3 mg/kg body weight by daily intramuscular injection starting postoperative day 1 for two weeks as experimental group. The rats were allowed for free cage activity during the experimental period. The femurs from ten rats of each group were allowed for histologic examination, the other fifteen femurs of each group were allowed for real time RT-PCR.
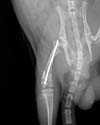
The rats were anesthetized with an intarperitoneal injection of ketamine (60 mg/kg). The skin, subcutaneous tissue and the capsule of the knee joint was incised under aseptic condition. Through a medial parapatellar approach, a 0.045-in nonthreaded Kirschner wire was inserted by electrical drill into each femoral canal by retrograde manner without reaming (Fig. 1). After insertion, the proximal end of wire was identified by palpation and it was confirmed the intramedullary position by radiography. The pinned femurs were placed in abduction and external rotation position in a three point bending apparatus and the fractures were created mechanically under displacement control.
The rats were sacrificed at postoperative two weeks by injecting a lethal dose of ketamine, since expression of MMP-13 reached experimentally to the maximum level at postfracture day 1421). The fractured femurs were removed and calluses were harvested for RNA extraction and histologic investigation.
Harvested calluses were fixed with 10% formalin solution for 24 hours, and then decalcified in 50% formic acid for 48 hours. Specimens were embedded in paraffin, were sagittally sectioned at 4 µm thickness and stained with hematoxylin and eosin. Each specimen was examined in order to determine if there were any differences between two groups regard to the number of osteoblast, the array pattern of fibrous tissue and the amount of callus.
The calluses obtained from fifteen rats of each group were prepared for RT-PCR. Primers were designed using Primer Express 2.0 (Biosystems, Foster City, CA, USA) software and are listed in Table 1. Template for the standard curves was generated via conventional PCR (GeneAmp PCR system 2400, Perkin Elmer, Foster City, CA, USA) using 1~5 ng of genomic DNA from the callus. PCR amplicons for standard curve were prepared by purification and ten-fold serial dilutions of a known concentration of amplicons. Total RNA from callus was extracted using RNAqueous-4PCR kit (Ambion, Austin,TX, USA) following manufacturer's directions. For the synthesis of cDNA, Reverse transcription was performed with first Strand cDNA Synthesis Kit for RT-PCR (Roche Diagnostic Corp., Indianapolis, IN, USA).
Real-time PCR was performed in a ABI PRISM® 7000 (Applied Biosystems) and melting curve analysis. Formation of expected PCR products was confirmed by agarose gel electrophoresis and melting curve analysis. During every cycle, fluorescence values were recorded and represented the amount of product amplified to that point in the amplification reaction. The more template at the beginning of the reaction, the fewer number of cycles it took to reach a point in which the fluorescent signal was first recorded as statistically significant above background. This point was defined as the threshold cycle (CT) and always occurred during the exponential phase of amplification.
Relative quantification of genes was determined for each callus in three separate PCR reactions using the same RNA preparation. The concentration of each gene (MMP-13 and GAPDH) was calculated by reference to the respective standard curve with the aid of the ABI PRISM® 7000 software. Relative gene expression was calculated as a ratio of target gene (MMP-13) concentration to housekeeping gene (GAPDH) concentration17).
There were no significant differences in gross findings between two groups. The fracture site of all specimens were covered with chondroid calluses. However, the experimental group demonstrated significantly more fibrous tissue and less new bone formation than the control group in histological assessment of the calluses (Fig. 2). Average number of osteoblasts was observed 24.5 in control group and 13.8 in experimental group under high powers of the microscope (p<0.05). Although we could not quantify amount of the fibrous tissue, the experimental group was observed much more fibrous tissue than the control group.
Relative gene expression was calculated as a ratio of target gene (MMP-13) concentration to housekeeping gene (GAPDH) concentration (Table 2). The mean expression of MMP-13 mRNA is 2.84±2.50 in the control group. The mean expression of MMP-13 mRNA is 1.16±1.05 in the experimental group. These results showed the significant difference between the control and experimental group by Student's t test (p<0.05).
The healing process of fracture is initiated by consecutive inflammatory cascade that contains various cytokines and growth factors. The representative products of inflammation are such as IL-1, IL-6, TNF and fibroblast growth factor4).
The process of bone formation must involve extensive proteolysis19) Transition of type II collagen to type I collagen is essential component in osteogenesis21). This transition is mediated by MMPs, especially MMP-13, that are one of the interstitial collagenases6). Hypertrophic chondrocyte and osteoblastic cells express MMP-13 mRNA in rats and human9). The main actions of this enzyme on fracture healing process are degradation of type II collagen and activation of osteoclast12). MMP-13 degrades type II collagen about six-fold more than type I and III collagen12). These findings suggested that MMP-13 is involved in initiation of resorption and remodeling of cartilagenous callus, promoting healing process. In contrast with effects on fracture healing, MMP-13 in rheumatoid arthritis has deleterious effects on disease progression18). Degradation of type II collagen, major component of cartilage, is associated with disease progression18,20). In rheumatoid arthritis, activated immune cells producing IL-1 and TNF mediate joint destruction. MMP-13 is induced in response to IL-1 and TNF. As a consequence, MMP-13 is considered to be therapeutic target in rheumatoid arthritis20). Therefore, MMP-13 has opposite enzymatic activity in fracture healing and rheumatoid arthritis.
The enzymatic reaction of cyclooxygenase is activated by two isoenzymes, COX-1 and COX-2. COX-1 is known to be essential in physiologic maintenance16). In contrast, COX-2 is known to be involved in pathophysiologic processes, such as inflammation, pain and fever13,15). But COX-2 in fracture healing produces proinflammatory prostaglandins which are necessary for induction of healing process3,5,8). Traditional NSAIDs have been reported to inhibit both cyclooxygenases. Many investigators reported the harmful effects of NSAIDs on fracture healing2,10,14). COX-2 inhibitor has equivalent analgesic efficacy compared with traditional NSAIDs. Also, they have been reported to lessen the side effects of NSAIDS such as gastrointestinal bleeding and platelet dysfunction3).
Simon et al.18) demonstrated that COX-2 function was important for fracture healing by COX-2 knockout mice. Although there are some studies that selective COX-2 inhibitor does not have a significant long-term effect on bone healing1,4,9), a number of studies have shown that COX-2 inhibitors have adverse effect on early fracture healing phase2,5,8,10,14,17).
In this study, the expression of MMP-13 mRNA was markedly decreased in COX-2 inhibitor treated group. It may be postulated that COX-2 inhibitor may delay fracture healing by suppressing the expression of MMP-13.
One limitation of our study is that we did not detect MMP-13 protein using the immunohistochemistry or wetern blotting, and so need further study. Another potential limitation is that the expression of MMP-13 mRNA could not represent entire enzymatic activity of MMP-13. But many MMPs are activated by other protease and organomercurials such amino phenyl mercuric acetate (APMA)11), which suggest the possibility of activation. No matter what the mechanism of decreased expression of MMP-13 mRNA is, COX-2 inhibitor suppresses the MMP-13 mRNA. Although no data of COX-2 inhibitor suggested an adverse effect on fracture healing in human, adverse effect must be considered especially in early phase of fracture healing.
Although the interaction between MMP-13 and COX-2 inhibitor is still unclear, our experiment shows that the expression of MMP-13 mRNA in COX 2 inhibitor injected rat group is suppressed and demonstrates significantly less new bone formation than non-injected rat group. Our result can not directly extrapolate to the fracture healing mechnism of human, but we can postulate the adverse effects of COX-2 inhibitor through this experiment. It is suggested that further study is mandatory to clarify the interaction between COX-2 inhibitor and MMP-13.
Figures and Tables
References
1. Akritopoulos P, Papaioannidou P, Hatzokos I, et al. Parecoxib has non-significant long-term effects on bone healing in rats when administered for a short period after fracture. Arch Orthop Trauma Surg. 2009; 129:1427–1432.

2. Altman RD, Latta LL, Keer R, Renfree K, Hornicek FJ, Banovac K. Effect of nonsteroidal antiinflammatory drugs on fracture healing: a laboratory study in rats. J Orthop Trauma. 1995; 9:392–400.
3. Bonnarens F, Einhorn TA. Production of a standard closed fracture in laboratory animal bone. J Orthop Res. 1984; 2:97–101.

4. Brown KM, Saunders MM, Kirsch T, Donahue HJ, Reid JS. Effect of COX-2-specific inhibition on fracture-healing in the rat femur. J Bone Joint Surg Am. 2004; 86:116–123.

5. Dunstan CR, Boyce R, Boyce BF, et al. Systemic administration of acidic fibroblast growth factor (FGF-1) prevents bone loss and increases new bone formation in ovariectomized rats. J Bone Miner Res. 1999; 14:953–959.

6. Endo K, Sairyo K, Komatsubara S, et al. Cyclooxygenase-2 inhibitor inhibits the fracture healing. J Physiol Anthropol Appl Human Sci. 2002; 21:235–238.

7. Freije JM, Diez-Itza I, Balbin M, et al. Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J Biol Chem. 1994; 269:16766–16773.

8. Gack S, Vallon R, Schmidt J, et al. Expression of interstitial collagenase during skeletal development of the mouse is restricted to osteoblast-like cells and hypertrophic chondrocytes. Cell Growth Differ. 1995; 6:759–767.
9. Gerstenfeld LC, Thiede M, Seibert K, et al. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2003; 21:670–675.

10. Henriet P, Rousseau GG, Eeckhout Y. Cloning and sequencing of mouse collagenase cDNA. Divergence of mouse and rat collagenases from the other mammalian collagenases. FEBS Lett. 1992; 310:175–178.

11. Holliday LS, Welgus HG, Fliszar CJ, Veith GM, Jeffrey JJ, Gluck SL. Initiation of osteoclast bone resorption by interstitial collagenase. J Biol Chem. 1997; 272:22053–22058.

12. Kherif S, Lafuma C, Dehaupas M, et al. Expression of matrix metalloproteinases 2 and 9 in regenerating skeletal muscle: a study in experimentally injured and mdx muscles. Dev Biol. 1999; 205:158–170.

13. Knäuper V, López-Otin C, Smith B, Knight G, Murphy G. Biochemical characterization of human collagenase-3. J Biol Chem. 1996; 271:1544–1550.

14. Lee SH, Soyoola E, Chanmugam P, et al. Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J Biol Chem. 1992; 267:25934–25938.

15. Sato H, Takino T, Okada Y, et al. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994; 370:61–65.

16. Schwartz JI, Chan CC, Mukhopadhyay S, et al. Cyclooxygenase-2 inhibition by rofecoxib reverses naturally occurring fever in humans. Clin Pharmacol Ther. 1999; 65:653–660.

17. Seibert K, Masferrer JL. Role of inducible cyclooxygenase (COX-2) in inflammation. Receptor. 1994; 4:17–23.
18. Simon AM, Manigrasso MB, O'Connor JP. Cyclo-oxygenase 2 function is essential for bone fracture healing. J Bone Miner Res. 2002; 17:963–976.

19. Ståhle-Bäckdahl M, Sandstedt B, Bruce K, et al. Collagenase-3 (MMP-13) is expressed during human fetal ossification and re-expressed in postnatal bone remodeling and in rheumatoid arthritis. Lab Invest. 1997; 76:717–728.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download