Abstract
Purpose
The purpose of this study was to examine womens' perceptions regarding influenza vaccination during pregnancy among Korean childbearing women.
Methods
Data was collected using focus group interviews from June to September, 2010. Forty Korean women participated in 13 focus groups. After obtaining permission from participants, each session of the focus group was audio-taped and transcribed verbatim. The responses were analyzed utilizing qualitative content analysis.
Results
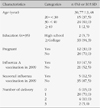
Forty women ranging from 26 to 43 participated in the study. The major themes were safety concerns; effects of fetal protection and infection prevention; lack of perceived needs; and encouragement as well as concerns from others. Participants raised questions on whether the vaccine was safe and effective, and concerns about the potential harmful effect of influenza vaccine to their bodies and the fetus. The major reason for influenza vaccination during pregnancy was to protect self and fetus. Also, clinician's recommendation was the facilitating factors for influenza vaccination during pregnancy.
References
1. Al-Tawfiq J.A., Antony A., Abed M.S. Attitudes towards influenza vaccination of multi-nationality healthcare workers in Saudi Arabia. Vaccine. 2009. 27(40):5538–5541.
2. Archer B., Cohen C., Naidoo D., Thomas J., Makunga C., Blumberg L., et al. Interim report on pandemic H1N1 influenza virus infections in South Africa, April to October 2009: Epidemiology and factors associated with fatal cases. Euro Surveillance. 2009. 14(42):pii: 19369.
3. Beigi R.H., Wiringa A.E., Bailey R.R., Assi T.M., Lee B.Y. Economic value of seasonal and pandemic influenza vaccination during pregnancy. Clinical Infectious Diseases. 2009. 49(12):1784–1792.
4. Benowitz I., Esposito D.B., Gracey K.D., Shapiro E.D., Vázquez M. Influenza vaccine given to pregnant women reduces hospitalization due to influenza in their infants. Clinical Infectious Diseases. 2010. 51(12):1355–1361.
5. Bish A., Michie S. Demographic and attitudinal determinants of protective behaviours during a pandemic: A review. British Journal of Health Psychology. 2010. 15(Pt 4):797–824.
6. Centers for Disease Control and Prevention (CDC). Receipt of influenza vaccine during pregnancy among women with live births--Georgia and Rhode Island, 2004-2007. MMWR. Morbidity and Mortality Weekly Report. 2009a. 58(35):972–975.
7. Centers for Disease Control and Prevention (CDC). Intent to receive influenza A (H1N1) 2009 monovalent and seasonal influenza vaccines-two counties, North Carolina, August 2009. MMWR. Morbidity and Mortality Weekly Report. 2009b. 58(50):1401–1405.
8. Centers for Disease Control and Prevention (CDC). Seasonal influenza and 2009 H1N1 influenza vaccination coverage among pregnant women--10 states, 2009-10 influenza season. MMWR. Morbidity and Mortality Weekly Report. 2010. 59(47):1541–1545.
9. Chapman G.B., Coups E.J. Predictors of Influenza Vaccine Acceptance among Healthy Adults. Preventive Medicine. 1999. 29(4):249–262.
10. Choi B.D. Pandemic influenza (H1N1 2009) infections among Korean pregnant women. 2010. Busan: Inje University;Unpublished master thesis.
11. Creanga A.A., Johnson T.F., Graitcer S.B., Hartman L.K., Al-Samarrai T., Schwarz A.G., et al. Severity of 2009 pandemic influenza A (H1N1) virus infection in pregnant women. Obstetrics and Gynecology. 2010. 115(4):717–726.
12. Fabry P., Gagneur A., Pasquier J.C. Determinants of A (H1N1) vaccination: Cross-sectional study in a population of pregnant women in Quebec. Vaccine. 2011. 29(9):1824–1829.
13. Jamieson D.J., Honein M.A., Rasmussen S.A., Williams J.L., Swerdlow D.L., Biggerstaff M.S., et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009. 374:451–458.
14. Kee S.Y. Influenza vaccine coverage rates and perceptions on vaccination in Korea. 2005. Seoul: Korea University;Unpublished master thesis.
15. Kim M., Lee S., Lee K., Kim A., Son D., Chung M., et al. Influenza vaccine coverage rate and related factors on pregnant women. Infection and Chemotherapy. 2009. 41(6):349–354.
16. Guidelines for influenza A prevention and management. Korea Center for Disease Control and Prevention. 2009. Retrieved September 15, 2009. from http://flu.cdc.go.kr.
17. Krueger R., Casey M. Focus Groups - A Practical Guide for Applied Research. 2000. 3rd ed. Thousand Oaks, CA: Sage.
18. Kwon Y., Cho H.Y., Lee Y.K., Bae G.R., Lee S.G. Relationship between intention of novel influenza A (H1N1) vaccination and vaccination coverage rate. Vaccine. 2010. 29(2):161–165.
19. Lau J.T., Cai Y., Tsui H.Y., Choi K.C. Prevalence of influenza vaccination and associated factors among pregnant women in Hong Kong. Vaccine. 2010. 28(33):5389–5397.
20. Lim S.H., Lee J.H., Kim B.C., Jung S.U., Park Y.B., Lee C.S. Adverse reaction of influenza A (H1N1) 2009 virus vaccination in pregnant women and its effect on newborns. Vaccine. 2010. 28(47):7455–7456.
21. Lincoln Y., Guba E. Naturalistic Inquiry. 1985. Beverly Hills, CA: Sage.
22. Michaelis M., Doerr H.W., Cinatl J. Jr. Novel swine-origin influenza A virus in humans: Another pandemic knocking at the door. Medical Microbiology and Immunology. 2009. 198(3):175–183.
23. Naleway A.L., Smith W.J., Mullooly J.P. Delivering influenza vaccine to pregnant women. Epidemiologic Reviews. 2006. 28:47–53.
24. Norton S.P., Scheifele D.W., Bettinger J.A., West R.M. Influenza vaccination in paediatric nurses: Cross-sectional study of coverage, refusal, and factors in acceptance. Vaccine. 2008. 26(23):2942–2948.
25. Ottenberg A.L., Wu J.T., Poland G.A., Jacobson R.M., Koenig B.A., Tilburt J.C. Vaccinating health care workers against influenza: The ethical and legal rationale for a mandate. American Journal of Public Health. 2011. 101(2):212–216.
26. Puleston R.L., Bugg G., Hoschler K., Konje J., Thornton J., Stephenson I., et al. Observational study to investigate vertically acquired passive immunity in babies of mothers vaccinated against H1N1v during pregnancy. Health Technology Assessment. 2010. 14(55):1–82.
27. Tamma P.D., Ault K.A., del Rio C, Steinhoff M.C., Halsey N.A., Omer S.B. Safety of influenza vaccination during pregnancy. American Journal of Obstetrics and Gynecology. 2009. 201(6):547–552.
28. White S.W., Petersen R.W., Quinlivan J.A. Pandemic (H1N1) 2009 influenza vaccine uptake in pregnant women entering the 2010 influenza season in Western Australia. The Medical Journal of Australia. 2010. 193(7):405–407.
29. Zaman K., Roy E., Arifeen S.E., Rahman M., Raqib R., Wilson E., et al. Effectiveness of maternal influenza immunization in mothers and infants. The New England Journal of Medicine. 2008. 359(15):1555–1564.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download