Abstract
Purpose
The p53 gene is located on chromosome 17p13 and may play important roles in cell cycle regulation, apoptosis and the regulation of the expression of other genes as well as tumor suppression. In addition, the p53 gene is believed to play an important role in the progression of various human malignant tumors through mutation and overexpression. There have been few studies on loss of heterozygosity (LOH) study on 17p13 in invasive ductal carcinoma. This study evaluated a 17p13 LOH and protein expression in invasive ductal carcinomas and correlated these results with the clinicopathological factors.
Methods
LOH analysis was carried out using a polymerase chain reaction with four polymorphic microsatellite markers (D17S796, TP53, D17S5, D17S513) in 50 surgically resected tumors and their non-tumorous counterparts. The p53 protein expression level was examined using immunohistochemistry.
Results
A LOH and protein expression was detected in 66% and 54% of the tumors, respectively. The LOH rates ranged from 26.3% (D17S513) to 33.3% (TP53). There was no detected LOH or protein expression in the non-tumor parts. The LOH results correlate well with the tumor size and stage. The protein expression results correlate well with the tumor histological grade. There was no correlation between the LOH and protein loss.
Conclusion
17p13 LOH and p53 gene abnormalities may be associated with tumorigenesis and tumor invasion. In addition, the combined use of both methods may help in early detection as well as for determining the prognosis of an invasive ductal carcinoma. 17p13 LOH and p53 protein expression may contribute to tumor progression through reciprocal complementation in some portions of the invasive ductal carcinoma.
Figures and Tables
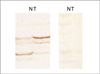 | Fig 1LOH at chromosome 17p13 loci in invasive ductal carcinoma. Representative D17S513 (left) and D17S5 microsatellite analysis of N (normal) and T (Tumor). Each T lane shows lower band loss (left) and considerable band loss (right). |
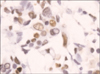 | Fig 2Immunohistochemically, the tumor cells of invasive ductal carcinoma show strong nuclear expression. |
References
1. 2002 Annual Report of the Central Cancer Registry in Korea. 2003. Republic of Korea: Ministry of Health and Welfare;11.
2. Sparano JA, Fazzari MJ, Childs G. Clinical application of molecular profiling in breast cancer. Future Oncol. 2005. 1:485–496.

3. Abrial C, Mouret-Reynier MA, Cure H, Feillel V, Leheurteur M, Lemery S, et al. Neoadjuvant endocrine therapy in breast cancer. Breast. 2006. 15:9–19.

5. Hollingsworth AB, Singletary SE, Morrow M, Francescatti DS, O'Shaughnessy JA, Hartman AR, et al. Current comprehensive assessment and management of women at increased risk for breast cancer. Am J Surg. 2004. 187:349–362.

6. Calderon-Margalit R, Paltiel O. Prevention of breast cancer in women who carry BRCA1 or BRCA2 mutations: a critical review of the literature. Int J Cancer. 2004. 10:357–364.
7. Kaptain S, Tan LK, Chen B. Her-2/neu and breast cancer. Diagn Mol Pathol. 2001. 10:139–152.
8. Ingvarsson S. Molecular genetics of breast cancer progression. Semin Cancer Biol. 1999. 9:277–288.

9. Liu MC, Gelmann EP. P53 gene mutations: case study of a clinical marker for solid tumors. Semin Oncol. 2002. 29:246–257.

10. Vousden KH, Prives C. P53 and prognosis: new insights and further complexity. Cell. 2005. 14:7–10.
11. Ryan KM, Phillips AC, Vousden KH. Regulation and function of the p53 tumor suppressor protein. Curr Opin Cell Biol. 2001. 13:332–337.

12. Soussi T. The p53 tumor suppressor gene: from molecular biology to clinical investigation. Ann N Y Acad Sci. 2000. 910:121–137.

13. Nicolini A, Carpi A, Tarro G. Biomolecular markers of breast cancer. Front Biosci. 2006. 11:1818–1843.

14. Hall PA, McCluggage WG. Assessing p53 in clinical contexts: unlearned lessons and new perspectives. J Pathol. 2006. 208:1–6.

15. Viktorsson K, De Petris L, Lewensohn R. The role of p53 in treatment responses of lung cancer. Biochem Biophys Res Commun. 2005. 331:868–880.

16. Morris SM. A role for p53 in the frequency and mechanism of mutation. Mutat Res. 2002. 511:45–62.

17. Tsuji N, Furuse K, Asanuma K, Furuya M, Kondoh K, Kamagata C, et al. Mutations of the p53 gene and loss of heterozygosity at chromosome 17p13.1 are associated with increased survivin expression in breast cancer. Breast Cancer Res Treat. 2004. 87:23–31.

19. Laptenko O, Prives C. Transcriptional regulation by p53: one protein, many possibilities. Cell Death Differ. 2006. 13:951–961.

20. Park JH, Kim SJ, Choi UJ, Lee KM. Correlation of the Immunohistochemical Coexpression of p53 and HER-2/neu and the Prognosis of Breast Cancer. J Breast Cancer. 2005. 8:41–47.

21. Jo HJ, Yun KJ, Moon HB. Expression of p21, p53 and bcl-2 Proteins in Invasive Ductal Carcinoma of the Breast. Korean J Pathol. 2003. 37:393–399.
22. Kim JK, Song YJ, Cho SI, Ryu DH, Yun HY, Sung RH. Clinicopathologic Significance of p53 and c-erbB-2 Protein Expression in Breast Carcinoma. J Korean Surg Soc. 2002. 62:282–287.

23. Roncuzzi L, Brognara I, Baiocchi D, Amadori D, Gasperi-Campani A. Loss of heterozygosity at 17p13.3-ter, distal to TP53, correlates with negative hormonal phenotype in sporadic breast cancer. Oncol Rep. 2005. 14:471–474.

24. Johnson SM, Shaw JA, Walker RA. Sporadic breast cancer in young women: prevalence of loss of heterozygosity at p53, BRCA1 and BRCA2. Int J Cancer. 2002. 98:205–209.

25. Ding SL, Sheu LF, Yu JC, Yang TL, Chen BF, Leu FJ, et al. Abnormality of the DNA double-strand-break checkpoint/repair genes, ATM, BRCA1 and TP53, in breast cancer is related to tumour grade. Br J Cancer. 2004. 90:1995–2001.

26. Otis CN, Krebs PA, Albuquerque A, Quezado MM, San Juan X, Sobel ME, et al. Loss of heterozygosity of p53, BRCA1, VHL, and estrogen receptor genes in breast carcinoma: correlation with related protein products and morphologic features. Int J Surg Pathol. 2002. 10:237–245.

27. Seitz S, Poppe K, Fischer J, Nothnagel A, Estevez-Schwarz L, Haensch W, et al. Detailed deletion mapping in sporadic breast cancer at chromosomal region 17p13 distal to the TP53 gene: association with clinicopathological parameters. J Pathol. 2001. 194:318–326.

28. Querzoli P, Albonico G, di Iasio MG, Ferretti S, Rinaldi R, Cariello A, et al. Biophenotypes and survival of BRCA1 and TP53 deleted breast cancer in young women. Breast Cancer Res Treat. 2001. 66:135–142.

29. Gentile M, Bergman Jungestrom M, Olsen KE, Soderkvist P, Wingren S. p53 and survival in early onset breast cancer: analysis of gene mutations, loss of heterozygosity and protein accumulation. Eur J Cancer. 1999. 35:1202–1207.

30. Suh KS, Lee YH, Na SY, Park MI, Kim HS, Lee SK. Mutational and Loss of Heterozygosity Analysis of the p53 and PTEN Tumor Suppressor Genes in Breast Carcinoma. Korean J Pathol. 2005. 39:313–319.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download