Abstract
Purpose
Vascular endothelial growth factor (VEGF) signal transduction mainly depends on its binding to VEGF receptor 2 (VEGFR-2). VEGF downstream signaling proteins mediate several of its effects in cancer progression, including those on tumor growth, metastasis, and blood vessel formation. The activation of VEGFR-2 signaling is a hallmark of and is considered a therapeutic target for breast cancer. Here, we report a study of the regulation of the VEGFR-2 signaling pathway by a small molecule, isomangiferin.
Methods
A human breast cancer xenograft mouse model was used to investigate the efficacy of isomangiferin in vivo. The inhibitory effect of isomangiferin on breast cancer cells and the underlying mechanism were examined in vitro.
Results
Isomangiferin suppressed tumor growth in xenografts. In vitro, isomangiferin treatment inhibited cancer cell proliferation, migration, invasion, and adhesion. The effect of isomangiferin on breast cancer growth was well coordinated with its suppression of angiogenesis. A rat aortic ring assay revealed that isomangiferin significantly inhibited blood vessel formation during VEGF-induced microvessel sprouting. Furthermore, isomangiferin treatment inhibited VEGF-induced proliferation of human umbilical vein endothelial cells and the formation of capillary-like structures. Mechanistically, isomangiferin induced caspase-dependent apoptosis of breast cancer cells. Furthermore, VEGF-induced activation of the VEGFR-2 kinase pathway was down-regulated by isomangiferin.
Breast cancer is by far the most frequent cancer among women with an estimated 1.38 million new cases diagnosed every year [1]. Radical mastectomy and chemotherapy are beneficial for most patients with localized breast cancer (approximately 85%–90%), with survival extending beyond 5 years. Unfortunately, for patients with distant metastasis, the survival rate is only approximately 10% to 18% [2]. Cure of late advanced stage breast cancer is relatively rare.
Vascular endothelial growth factor receptor 2 (VEGFR-2) is a cell membrane-bound tyrosine kinase receptor that was initially discovered in endothelial cells. VEGFR-2 plays a key role in the development of tumor angiogenesis [3]. Although VEGFR-2 was originally described as being exclusively expressed in endothelial cells, many studies have demonstrated that it is also expressed in tumor cells from patients with colon cancer [4], pancreatic cancer [5], lung cancer [6], cervical adenosquamous carcinoma [7], medullary thyroid carcinoma [8], glioma [9], oral squamous cell carcinoma [10], and breast cancer [11]. Upregulation of VEGFR-2 mRNA is detected was in the early stages of invasive primary and metastatic breast cancers. A strong correlation has been demonstrated between the expression of VEGFR-2 and the clinicopathological outcomes in breast cancer. VEGFR-2 expression has also been positively correlated with lymph node metastasis in breast cancer, with high expression of VEGFR-2 associated with significantly worse survival outcomes [12]. Furthermore, in breast cancer, the expression of epithelial-mesenchymal transition (EMT) markers, including Twist1 and vimentin, are higher in tumors displaying a higher expression of VEGFR-2, while E-cadherin expression is lower in the same tumors, suggesting that VEGFR-2 may serve as a potential mediator of EMT in breast cancer [13]. Therefore, blocking the VEGFR-2 signaling pathway is promising for breast cancer therapy.
Isomangiferin is a xanthone C-glucoside that is a component of many plants in the Cyclopia subfamily such as Coffea arabica and Anemarrhenae rhizoma [14]. While isomangiferin has cytotoxic activity on some cancer cells [15] its effect on breast cancer has not been adequately explored. Herein, we examined the potential anti-cancer activity of isomangiferin on breast tumor growth, metastasis, and angiogenesis, and the underlying mechanism(s).
Isomangiferin was purchased from Shanghai Yuan Ye Biotechnology Co., Ltd. (Shanghai, China). Its purity was verified as ≥98% by high-performance liquid chromatography. Reagents used for cell cultures such as fetal bovine serum (FBS) and trypsin, and the growth medium were all purchased from Gibco Life Technology (Carlsbad, USA). Antibodies specific for actin, VEGFR-2, phosphorylated VEGFR-2 (p-VEGFR-2), platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31), protein kinase B (PKB/AKT), phosphorylated protein kinase B (p-AKT), extracellular regulated protein kinase (ERK), phosphorylated ERK (p-ERK), poly ADP-ribose polymerase (PARP), cleaved PARP, phosphorylated signal transducer and activator of transcription 3 (p-STAT3), focal adhesion kinase (FAK), Caspase 3, and β-actin were obtained from Cell Signaling Technology (Danvers, USA). The MTS cell proliferation assay kit was purchased from BD Biosciences (Franklin Lakes, USA). VEGFR-2 signaling pathway agonist vascular endothelial growth factor (VEGF) and antagonist SU5408 were purchased from Sigma-Aldrich (St. Louis, USA).
Human breast carcinoma cell lines used for in vitro experiments included MDA-MB-231, T47D, MCF7, and SKBR3. The 4T1 triple-negative mouse breast cancer cell line was also used. The MCF-10A immortalized normal human mammary epithelial cell line was used as a normal control to test the cancer cell cytotoxicity of isomangiferin. All cells were purchased from the American Type Culture Collection (Manassas, USA) and cultured in high-glucose Dulbecco's modified Eagle's medium or Ham's F12K (Kaighn's) medium containing 10% FBS in an atmosphere of 5% CO2 at 37℃. Primary human umbilical vein endothelial cells (HUVECs; ScienCell Research Laboratories, San Diego, USA) were used as a model to mimic the process of angiogenesis. The HUVECs were cultured in Endothelial Cell Medium (Gibco Life Technology) supplemented with 5% FBS, 1% endothelial cell growth supplement, and 1% penicillin-streptomycin in an atmosphere of 5% CO2. The culture medium was refreshed every 2 to 3 days.
MDA-MB-231 cells were used for the mouse breast cancer mouse xenograft model. Five-week-old male BALB/c nude mice with body weights of approximately 25 g were randomly divided into two groups. MDA-MB-231 cells were injected subcutaneously into each mouse (2×106 cells per mouse). When the average volume of each tumor was approximately 100 mm3, mice were left untreated or treated with isomangiferin (10 mg/kg/day) each day for a month by intraperitoneal injection. During the administration of isomangiferin, the tumor growth of MDA-MB-231 xenografts was monitored every 6 days. Thirty days later, all mice were sacrificed and dissected. The solid subcutaneous tumors were recovered for photographic imaging and weighing.
The dissected tumors were also used for histology and immunohistochemistry analyses. Tumor tissue was fixed with 10% formaldehyde for 24 hours followed by conventional dehydration. Paraffin-embedded tumors were carefully sectioned to a thickness of 4 µm and then stained with antibody to p-VEGFR-2 and CD31. The stained cells were analyzed by Image-Pro Plus software (Media Cybernetics Inc., Rockville, USA).
Cells (5,000/well) were seeded in 96-well plates for 48 hours as previously described [161718] and treated with 1 µM of isomangiferin. Cell viability was assessed using an MTS assay kit (Promega, Madison, USA) as detailed by the manufacturer. The absorbance value of the live cells in the wells of the 96-well plates was measured at 515 nm using a microplate reader (Thermo Fisher Scientific, Waltham, USA). For the SU5408 treatment, MDA-MB-231 cells were first incubated with 100 nM SU5408 and then treated with the indicated concentrations of isomangiferin.
To determine the inhibitory effect of isomangiferin on MDA-MB-231 cell migration, the Boyden chamber assay was performed. MDA-MB-231 cells (4×104/well) were starved prior to the experiment in 100 µL of medium deprived of FBS. Isomangiferin was added to the upper chambers (8 µm; BD Biosciences) at different concentrations along with 50 ng/mL VEGF. The bottom wells were filled with 600 µL medium containing 0.5% FBS. After cells were treated for 4 to 6 hours to allow for migration of cells from the upper to the lower chamber. The cells were fixed with 4% paraformaldehyde for more than 30 minutes. Non-migrating cells that remained in the upper chamber were gently removed using a cotton swab and the cells that had migrated to the bottom chamber were stained with 1% crystal violet. The protocol for the cell invasion assay was almost the same as for the migration assay, except that the membrane of the upper chamber was pretreated with 0.1% gelatin. Images were photographed using an inverted microscope (Olympus Life Science, Tokyo, Japan). The cells that had migrated to the bottom chamber were enumerated in five randomly selected fields.
Dissected rat thoracic aortas were cut into rings with a diameter of 1–1.5 mm and randomly seeded in Matrigel-coated wells. Thin rings were covered with another 100 µL of Matrigel and allowed to solidify for 30 minutes, then fresh medium without serum was added. Twenty-four hours later, the medium was replaced with fresh medium containing 2.5% FBS without isomangiferin or with different concentrations of isomangiferin. The medium was refreshed every other day. After 5 days, microvessel sprouts were photographed using an inverted microscope (Olympus Life Science).
Fifty microliters of Matrigel (BD Biosciences) per well was added to the wells of pre-cooled 96-well plates and polymerized at 37℃ for 30 minutes. HUVECs (6.5×103/well) were seeded into the Matrigel-coated plates and treated with various concentrations of isomangiferin containing 50 ng/mL VEGF after being starved for 6 hours. Culture medium without VEGF was used as the negative control. After 10 hours, tubulogenesis was recorded by photography and the tubular structures were counted manually.
The Apoptosis Detection Kit (BD Biosciences) was used to test the induction of apoptosis in breast cancer cells by isomangiferin. After treatment with isomangiferin for 36 hours, MDA-MB-231 cells were collected, washed, and stained with annexin V-fluorescein isothiocyanate and propidium iodide and were analyzed by flow cytometry using a FACSCalibur apparatus (BD Biosciences) as detailed elsewhere [19].
The wells of 24-well plates were pre-coated with 0.04 µg/µL Matrigel mixed with FBS-free medium overnight. MDA-MB-231 cells (approximately 106) added to each well and allowed to reach 90% confluence. Prior to use, the cells were washed with medium to remove serum. For the isomangiferin adhesion inhibition assay, the MDA-MB-231 cells were incubated with the indicated concentrations of isomangiferin for 1 hour. Following incubation, the cells adhered to the Matrigel coating in the wells. Nonadhering cells were removed by washing three times with phosphate buffered saline. After fixation with 4% paraformaldehyde and staining with 1% crystal violet, the number of adhering cells was quantified using the aforementioned inverted microscope.
VEGFR-2 kinase activity (CST HTScan®, Cat No. #7788) was performed using a VEGFR-2 kinase assay kit purchased from Cell Signaling Technology. Briefly, the kinase activity was measured in a radiometric assay using the following reaction ingredients: 4 mM 3-(N-morpholino) propanesulfonic acid (pH 7.2), 2.5 mM β-glycerophosphate, 1 mM ethylene glycol-bis-(2-aminoethyl ether)-N,N′-tetraacetic acid, 0.4 mM ethylenediamine tetraacetic acid (EDTA), 4 mM magnesium chloride hexahydrate, 0.05 mM dithiothreitol, and 50 µM adenosine triphosphate. The substrates were recombinant human myelin basic protein (200 ng/µL) and variable amounts of recombinant human VEGFR-2. SU5408 and VEGFR-2 kinase inhibitor I were employed as positive controls.
MDA-MB-231 cells and HUVECs were pre-treated in the absence or presence of various concentrations of isomangiferin for 1 to 2 hours. The cells were then stimulated with VEGF (100 ng/mL) for 5 to 20 minutes. Total protein was extracted using RIPA lysis buffer (50 mM Tris–HCl, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 1% sodium deoxycholic acid, 0.1% sodium dodecyl sulfate (SDS), 2 mM phenylmethylsulfonyl fluoride, 30 mM Na2HPO4, 50 mM NaF, and 1 mM Na3VO4 for 30 minutes on ice as detailed elsewhere [2021]. An equal quantity of protein from each sample was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and the resolved proteins were, transferred to nitrocellulose filter membranes. Blots were incubated with specific antibodies following blockage for 1 hour at room temperature with 5% FBS. Finally, blots were examined by LI-COR Infrared Imaged Odyssey (Gene Company Ltd., Hong Kong, China).
All the statistical results were analyzed using the Student t-test, except for the statistical analysis of tumor weight, which was conducted using two-way ANOVA performed with GraphPad Prism 5 (GraphPad Software Inc., La Jolla, USA) to compare group means. Each experiment was performed independently in triplicate. A value of p<0.05 represented statistical significance.
The MDA-MB-231 xenograft breast tumor model was used to investigate the effect of isomangiferin on tumor growth or angiogenesis in vivo. Tumor volume was recorded during the treatment process. As shown in Figure 1A and 1B, compared with that of controls, growth of xenografts in the isomangiferin-treated group was slower. Statistical analysis revealed a significant difference between the control group and the isomangiferin-treated group (p<0.01) (Figure 1C), indicating that isomangiferin treatment could suppress breast tumor growth in vivo. Notably, the tumors in the control group had tiny blood vessels compared to the tumor blood vessels in the isomangiferin-treated group (dark red tumors in upper panel of Figure 1A vs. pale-appearing tumors in the bottom panel of Figure 1A). We anticipated that isomangiferin might inhibit tumor angiogenesis, which could markedly affect tumor growth. A VEGFR-2 antibody was used to stain solid tumor sections to examine tumor angiogenesis. As shown by the immunohistochemistry result in Figure 1D, the intensity of p-VEGFR-2 staining in the isomangiferin-treated group was markedly lower than that in the control group. The expression of CD31, a surface marker of neovascular endothelial cells, was accordingly reduced after isomangiferin administration. These results suggested that isomangiferin can partially inhibit tumor growth because of its inhibition of tumor angiogenesis.
We first tested the effect of 1 µM isomangiferin on breast cancer cells (MDA-MB-231, T47D, MCF7, SKBR3, 4T1) and human normal mammary epithelial cells (MCF-10A) using the MTS viability assay. As shown in Figure 2A, treating breast cancer cells with isomangiferin distinctly suppressed the cell viability compared to the MCF-10A control cells. The halfmaximal inhibitory concentration (IC50) of isomangiferin for the breast carcinoma cells was approximately 1 µM, while the IC50 for the MCF-10A cells exceeded 100 µM (data not shown), indicating that isomangiferin was more toxic to cancer cells than to normal cells. These findings indicate the selective effect of isomangiferin on breast cancer cells.
In breast cancer, metastasis-related recurrence is still common and is responsible for the cancer-associated mortality. To determine the effect of isomangiferin on breast cancer metastasis, we performed cell migration and invasion assays using MDA-MB-231 cells. These cells feature a highly malignant mobility. The results of the migration assay (Figure 2B) and invasion assay (Figure 2C) showed that as the dose of isomangiferin increased, the number of migrating and invading cells dramatically decreased. The collective results indicated that isomangiferin can inhibit the migration and invasive behaviors of MDA-MB-231 cells.
The rat aortic ring assay is a classical assay to study all steps in angiogenesis such as endothelial cell activation, pericyte acquisition, migration, and remodeling. Presently, the aortic ring assay was performed to investigate the anti-angiogenic effect of isomangiferin. As shown in Figure 3A, in the control group, a large number of microvessels sprouted from aortic rings on day 5 following the embedding of the rings in Matrigel. In contrast, isomangiferin significantly reduced microvessel sprouting, with microvessel formation almost completely blocked by 1 µM isomangiferin (Figure 3B). Endothelial cells spontaneously formed three-dimensional capillaries exposed to the Matrigel; this characteristic could be applied to mimic human angiogenesis. Using the three-dimensional vascular tube formation assay, we examined the effect of isomangiferin on tube-like structure formation of endothelial cells. HUVECs treated with different concentrations of isomangiferin were added to the surface of Matrigel. Vascular tubulogenesis was completely inhibited by isomangiferin at a concentration of 3 µM (Figure 3C and 3D). Taken together, these data suggest that isomangiferin suppresses the angiogenesis process of endothelial cells.
Flow cytometry analysis was performed on MDA-MB-231 cells to investigate whether isomangiferin could induce cell apoptosis. As shown in Figure 3E, isomangiferin treatment led to the induction of apoptosis in MDA-MB-231 cells in a dose-dependent manner. Notably, as the isomangiferin concentration increased from 0 to 5 µM, the percentage of apoptotic cells increased from 7.15% to 13.06%. We carried out an adhesion assay to examine the effect of isomangiferin on breast cancer cells. The result in Figure 3F reveals that isomangiferin impaired the attachment of live cancer cells to Matrigel, which imitated the progress of cancer cell adherence and penetration of the extracellular basement membrane of the tumor microenvironment. Taken together, the data demonstrate that isomangiferin has an inhibitory effect on the growth and adhesion of breast cancer cells.
The VEGFR-2 kinase assay was performed to investigate whether isomangiferin is a potential VEGFR-2 inhibitor for breast cancer therapy. SU5408, the classical cell-permeable inhibitor of VEGFR-2 kinase, had an IC50 of 72.42 nM, which was consistent with previous reports [22]. Isomangiferin also inhibited VEGFR-2 kinase activity with an IC50 of 820.67 nM (Supplementary Table 1, available online). Western blot examination revealed that isomangiferin treatment led to the activation of cleaved PARP and reduction of caspase 3 in MDA-MB-231 cells (Figure 4A). To further confirm that the isomangiferin-mediated suppression of breast cancer cells depended on VEGFR-2 signaling, cells were untreated or pretreated with 100 nM SU5408 prior to treatment with different concentrations of isomangiferin. As shown in Figure 4B, isomangiferin inhibited the proliferation of MDA-MB-231 cells in a dose-dependent manner (blue columns). In contrast, the proliferation of cells pretreated with SU5408 was not inhibited by isomangiferin (deep red columns). Examination of key signaling molecules involved in VEGFR-2-mediated signaling pathway upon isomangiferin treatment revealed that isomangiferin strongly blocked VEGF-induced VEGFR-2 phosphorylation at the Tyr1175 site in a concentration-dependent manner (Figure 4C). Isomangiferin suppressed the expression of p-AKT, phosphorylated extracellular regulated protein kinases (p-ERK), p-STAT3, and FAK, while the total amount of AKT or extracellular regulated protein kinases (ERK) were not influenced. These proteins are downstream molecules of VEGFR-2 and play critical roles in proliferation, migration, and differentiation of endothelial cells, indicating that isomangiferin inhibits angiogenesis through a VEGFR-2-mediated pathway.
The purpose of this study is to examine the therapeutic potential of VEGFR-2 for breast cancer. Our findings demonstrate that the natural molecule isomangiferin might have the potential to suppress human breast tumor growth, metastasis, and angiogenesis by targeting the VEGFR-2 signaling pathway.
Cancer cells secrete VEGF to activate the VEGFR-2 pathway, which in turn stimulates cancer-related proliferation, migration, invasion, and angiogenesis. Angiogenesis is especially important for the blood and nutrition supply when a tumor develops, so it plays an essential role in all steps of tumorigenesis including tumor growth, invasion, and metastasis. The VEGF signaling pathway is initiated when VEGF recognizes and binds to its receptor, the tyrosine kinase VEGFR. The signaling involved in angiogenesis is mainly mediated by VEGFR-2. Specifically, the activation of VEGFR-2 leads to further activation of many different intracellular signaling molecules, such as FAK, phosphoinositide 3-kinase/AKT kinase, mammalian target of rapamycin (mTOR)/ribosomal protein S6 kinase (p70S6K), protein kinase C/protein kinase D [23], ERK [24].
Since the VEGFR-2 signaling pathway is the hallmark of cancer, the United States Food and Drug Administration recently approved bevacizumab (marketed as Avastin®), a monoclonal antibody against VEGF, for the treatment of cancer. Importantly, this drug can be curative in many kinds of cancers [25]. Searching for safer, affordable, and efficacious small molecules with inhibitory activity against VEGFR-2 pathway is a hot area in cancer research.
Here, we demonstrate that isomangiferin inhibits the proliferation of several breast cancer cell lines in vitro, and the efficacy against breast cancer was demonstrated in vivo as well. In addition, isomangiferin concentrations as low as 1 µM were sufficient to impair the VEGF-induced angiogenic responses both in vitro and in vivo. At 3 µM, isomangiferin almost thoroughly blocked the capillary-like structure formation and microvessel sprouting process. Our results demonstrate that isomangiferin has anti-angiogenic activity that leads to the inhibition of tumor growth. We also show that isomangiferin treatment induces MDA-MB-231 cell apoptosis through a caspase-dependent pathway.
Isomangiferin inhibits breast cancer mainly by blocking the VEGFR-2-mediated angiogenesis pathway. In this study we not only verified that isomangiferin inhibited the VEGFR-2 pathway, but also proved that isomangiferin had direct impeding activity to block VEGFR-2's in vitro activity, thus might function as a tyrosine receptor kinase antagonist. Considerring VEGFR-2 is an important therapeutic target, isomangiferin may have great potential to be developed in same way like VEGFR-2 small molecular kinase inhibitors. However, there are some limitations to our study. We did not rule out other molecular mechanisms that could also contribute to isomangiferin's anti-cancer activity. For example, little is known about isomangiferin's anti-cancer activity compared to that of its analog, mangiferin. Thus, it can be inferred that isomangiferin and mangiferin might share some molecular mechanisms and pharmacological actions. For example, mangiferin can reduce the level of blood glucose and reduce cell nutrient metabolism [26]. We can logically speculate that isomangiferin may have some analogous function to decrease the malignancy of tumors through the downregulation of the cell glucose metabolism and nutrient intake. Furthermore, in a previous study, mangiferin caused cell cycle arrest of human promyelocytic leukemia HL60 cells by increasing the stability of the Nrf2 protein through inhibition of its ubiquitination and degradation [27]. Another study reported that mangiferin inhibits the invasion of LNCaP human prostate cancer cells by suppressing nuclear factor-κB and matrix metalloproteinase-9 [28]. Concerning isomangiferin's anti-cancer activity, relatively mature research experience with mangiferin provides a good example and a promising direction. In this study, isomangiferin inhibited tumor angiogenesis by blocking the kinase activity of VEGFR-2 as well as its downstream signals including p-AKT, p-ERK, and FAK. Interestingly, inhibiting tumor angiogenesis has also been revealed before in the potential role of mangiferin in cancer treatment [29]. Our results increase the awareness of isomangiferin's pharmacological mechanisms and reveal an innovative mechanism of isomangiferin's anti-cancer property.
In conclusion, the natural small molecule isomangiferin has the potential to inhibit breast tumor growth, metastasis, and angiogenesis, and it acts by targeting the VEGFR-2 signaling pathway. Our study provides evidence that isomangiferin has potential value in breast cancer treatment.
Figures and Tables
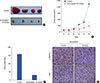 | Figure 1Isomangiferin inhibits breast tumor growth and vascular endothelial growth factor receptor 2 (VEGFR-2) signaling pathway in vivo. Human breast cancer cells MDA-MB-231 were injected subcutanously into 5-week-old BALB/cA nude mice (2×106 per mouse). When subcutanous tumors grew to about 100 mm3, the mice were intraperitoneally treated with or without isomangiferin (10 mg/kg/day). (A) Photos for isomangiferin treated or non-treated tumors. (B) Isomangiferin supressed MDA-MB-231 xenografts growth. Tumor volume was recorded every 6 days and the tumor growth curve was drafted through Graphpad Prism 5 software package. Values are shown as mean±standard error of the mean (SEM) of three independent experiments. (C) Isomangiferin inhibited breast tumor growth as measured by tumor weight. Values are shown as mean±SEM of three independent experiments. (D) Immunohistochemical staining revealed that isomangiferin inhibited VEGFR-2 signaling pathway by blotting phosphorylated VEGFR-2 (p-VEGFR-2) and reducing CD31 expression. Tumor sections from isomangiferin-treated and isomangiferin-untreated groups were stained using p-VEGFR-2 and CD31 antibodies, and the number of positive cells was counted (IHC stain for p-VEGFR-2, CD31, ×400).
*p<0.01 vs. control.
|
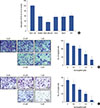 | Figure 2Isomangiferin inhibits breast cancer cell proliferation, migration and invasion. (A) Isomangiferin inhibited different types of breast cancer cells proliferation. Five variable kinds of breast cancer cells and one normal breast cell MCF-10A were treated with different doses of isomangiferin for 48 hours. Data are shown as mean±standard error of the mean (SEM) from triplicate experiments. The cell viability was determined by MTS assay according to Methods. (B) Isomangiferin supressed MDA-MB-231 migration. MDA-MB-231 cells were seeded in transwell chambers and then deprived of fetal bovine serum (FBS) for 6 hours. After that, cells were treated with 10 ng/mL vascular endothelial growth factor (VEGF) and indicated concentrations of isomangiferin. The migrated cells were quantified by manual counting by using an microscope (scale bar, 10 µm). Values are shown as mean±SEM of three independent experiments. (C) Isomangiferin imparied MDA-MB-231 invasion. After MDA-MB-231 cells seeded in the upper chamber of a transwell which has been pre-coated with Matrigel, the cells were deprived of FBS and then treated with different concentrations of isomangiferin. After 8 to 10 hours, the MDA-MB-231 cells that invaded through the membrane from the upper chamber to the bottom chamber were quantified when stimulated by the bottom chamber which was added VEGF (scale bar, 20 µm). Values are shown as mean±SEM of three independent experiments.
*p<0.05 vs. MCF-10A; †p<0.01 vs. MCF-10A; ‡p<0.05 vs. 0 µM isomangiferin; §p<0.01 vs. 0 µM isomangiferin; ∥p<0.001 vs. 0 µM isomangiferin.
|
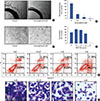 | Figure 3Isomangiferin inhibited the vascular endothelial growth factor (VEGF)-induced microvessel sprouting and tube formation of human umbilical vein endothelial cells (HUVECs) and induced MDA-MB-231 breast cancer cells apoptosis and adhension. (A) From Sprague-Dawley rats, the aortic segments were isolated and then were placed in growth factor-reduced Matrigel-covered wells. To stimulate angiogenesis, the vessel rings were treated with 50 ng/mL VEGF in the presence or absence of isomangiferin for 5 days. Representative photographs of endothelial cell sprouts forming branching cords from the margins of aortic rings under the treatment of 1 µM isomangiferin. (B) Sprouts were scored from 0 (least positive) to 5 (most positive) in a double-blinded manner. Values are shown as mean±standard error of the mean (SEM) of three independent experiments. (C) Isomangiferin inhibited the VEGF-induced tube formation of HUVEC tubes. HUVECs were placed in 96-well plates coated with Matrigel (6.5×103 per well). After 10 hours, cells were fixed, and tubular structures were photographed (magnification, ×100). (D) The statistic results of Figure 3C. Values are shown as mean±SEM of three independent experiments. (E) After incubation with different doses of isomangiferin for 36 hours, MDA-MB-231 cells were collected and apoptosis was assessed by annexin V-fluorescein isothiocyanate and prodidium iodide (annexin V/PI) staining and flow cytometry (n=3). (F) MDA-MB-231 cells were incubated with indicated doses of isomangiferin for 1 hour. Following incubation, cells were exposed and adhered to Matrigel. After 1% crystal violet stained, the number and phenotype of adhering cells was determined by microscope (magnification, ×400).
*p<0.05 vs. control; †p<0.01 vs. control; ‡p<0.001 vs. control.
|
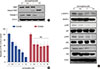 | Figure 4Isomangiferin suppresses breast cancer through inhibiting the vascular endothelial growth factor receptor 2 (VEGFR-2) signaling pathway. (A) Isomangiferin induced cell apoptosis. Proteins from MDA-MB-231 cells treated with indicated concentrations of isomangiferin for 24 hours were submitted to Western blot for the immunoblotting of caspase-3 and cleaved poly ADP-ribose polymerase (cleaved PARP). (B) Isomangiferin's ihibiton on breast cancer cell proliferation was dependent on VEGFR-2 activity. 100 nM SU5408 was used or not to block VEGFR-2's activity and then MDA-MB-231 cells were treated with isomangiferin. The cell proliferation was assessed by MTS assay. Values are shown as mean±standard error of the mean of three independent experiments. (C) Isomangiferin suppressed the activation of VEGFR-2 in human umbilical vein endothelial cells (HUVECs). The activation of VEGFR-2 was analyzed by Western blot and probed with indicated antibodies. Western blot was conducted in the way that described in Methods and specific antibodies were used accordingly.PARP=poly ADP-ribose polymerase; NS=not significant; p-AKT=phosphorylated protein kinase B, p-PKB/p-AKT; AKT=protein kinase B, PKB/AKT; p-ERK=phosphorylated extracellular regulated protein kinases; ERK=extracellular regulated protein kinases; p-STAT3=phosphorylated signal transducer and activator of transcription 3; FAK=focal adhesion kinase. *p<0.05 vs. control; †p<0.01 vs. control.
|
Notes
This work was supported by the National Natural Science Foundation of China (No. 81602649), Hubei Provincial Natural Science Foundation of China (No. 2016CFB210), Scientific Research Fund of Heilongjiang Provincial Education Department (No. 12541703) and the Startup Scientific Research Foundation for Doctors of Hubei University of Science and Technology (No. BK1506 and No. BK1525).
References
1. Jandial R, Hoshide R, Waters JD, Somlo G. Operative and therapeutic advancements in breast cancer metastases to the brain. Clin Breast Cancer. Epub 2017 Oct 7. DOI: 10.1016/j.clbc.2017.10.002.

2. Moreno AC, Lin YH, Bedrosian I, Shen Y, Stauder MC, Smith BD, et al. Use of regional nodal irradiation and its association with survival for women with high-risk, early stage breast cancer: a national cancer database analysis. Adv Radiat Oncol. 2017; 2:291–300.

3. Santoni M, Guerra F, Conti A, Lucarelli A, Rinaldi S, Belvederesi L, et al. Incidence and risk of cardiotoxicity in cancer patients treated with targeted therapies. Cancer Treat Rev. 2017; 59:123–131.

4. Zhou J, Wang H, Zhang H, Lutz AM, Tian L, Hristov D, et al. VEGFR2-targeted three-dimensional ultrasound imaging can predict responses to antiangiogenic therapy in preclinical models of colon cancer. Cancer Res. 2016; 76:4081–4089.

5. Pan Y, Zheng M, Zhong L, Yang J, Zhou S, Qin Y, et al. A preclinical evaluation of SKLB261, a multikinase inhibitor of EGFR/Src/VEGFR2, as a therapeutic agent against pancreatic cancer. Mol Cancer Ther. 2015; 14:407–418.

6. Maione P, Sgambato A, Casaluce F, Sacco PC, Santabarbara G, Rossi A, et al. The role of the antiangiogenetic ramucirumab in the treatment of advanced non small cell lung cancer. Curr Med Chem. 2017; 24:3–13.

7. Longatto-Filho A, Pinheiro C, Martinho O, Moreira MA, Ribeiro LF, Queiroz GS, et al. Molecular characterization of EGFR, PDGFRA and VEGFR2 in cervical adenosquamous carcinoma. BMC Cancer. 2009; 9:212.

8. Dunna NR, Kandula V, Girdhar A, Pudutha A, Hussain T, Bandaru S, et al. High affinity pharmacological profiling of dual inhibitors targeting RET and VEGFR2 in inhibition of kinase and angiogeneis events in medullary thyroid carcinoma. Asian Pac J Cancer Prev. 2015; 16:7089–7095.

9. Szabo E, Schneider H, Seystahl K, Rushing EJ, Herting F, Weidner KM, et al. Autocrine VEGFR1 and VEGFR2 signaling promotes survival in human glioblastoma models in vitro and in vivo. Neuro Oncol. 2016; 18:1242–1252.

10. Sato H, Takeda Y. VEGFR2 expression and relationship between tumor neovascularization and histologic characteristics in oral squamous cell carcinoma. J Oral Sci. 2009; 51:551–557.

11. Zhang J, Liu C, Shi W, Yang L, Zhang Q, Cui J, et al. The novel VEGF receptor 2 inhibitor YLL545 inhibits angiogenesis and growth in breast cancer. Oncotarget. 2016; 7:41067–41080.

12. Zhu X, Zhou W. The emerging regulation of VEGFR-2 in triple-negative breast cancer. Front Endocrinol (Lausanne). 2015; 6:159.

13. Yan JD, Liu Y, Zhang ZY, Liu GY, Xu JH, Liu LY, et al. Expression and prognostic significance of VEGFR-2 in breast cancer. Pathol Res Pract. 2015; 211:539–543.

14. Islam MN, Yoo HH, Lee J, Nam JW, Seo EK, Jin C, et al. Simultaneous determination of bioactive xanthone glycosides and norlignans from ethanolic extract of Anemarrhena asphodeloides by liquid chromatography. J AOAC Int. 2008; 91:1271–1277.

15. Satish Rao BS, Sreedevi MV, Nageshwar Rao B. Cytoprotective and antigenotoxic potential of Mangiferin, a glucosylxanthone against cadmium chloride induced toxicity in HepG2 cells. Food Chem Toxicol. 2009; 47:592–600.

16. Shen J, Song G, An M, Li X, Wu N, Ruan K, et al. The use of hollow mesoporous silica nanospheres to encapsulate bortezomib and improve efficacy for non-small cell lung cancer therapy. Biomaterials. 2014; 35:316–326.

17. Song G, Shen J, Jiang F, Hu R, Li W, An L, et al. Hydrophilic molybdenum oxide nanomaterials with controlled morphology and strong plasmonic absorption for photothermal ablation of cancer cells. ACS Appl Mater Interfaces. 2014; 6:3915–3922.

18. Li C, Hu J, Li W, Song G, Shen J. Combined bortezomib-based chemotherapy and p53 gene therapy using hollow mesoporous silica nanospheres for p53 mutant non-small cell lung cancer treatment. Biomater Sci. 2016; 5:77–88.

19. Shen J, Ma H, Zhang T, Liu H, Yu L, Li G, et al. Magnolol inhibits the growth of non-small cell lung cancer via inhibiting microtubule polymerization. Cell Physiol Biochem. 2017; 42:1789–1801.

20. Shen J, Sheng X, Chang Z, Wu Q, Wang S, Xuan Z, et al. Iron metabolism regulates p53 signaling through direct heme-p53 interaction and modulation of p53 localization, stability, and function. Cell Rep. 2014; 7:180–193.

21. Shen J, Zhang S, Li Y, Zhang W, Chen J, Zhang M, et al. p14(ARF) inhibits the functions of adenovirus E1A oncoprotein. Biochem J. 2011; 434:275–285.

22. Yamada KH, Kang H, Malik AB. Antiangiogenic therapeutic potential of peptides derived from the molecular motor KIF13B that transports VEGFR2 to plasmalemma in endothelial cells. Am J Pathol. 2017; 187:214–224.

23. Varga A, Gyulavári P, Greff Z, Futosi K, Németh T, Simon-Szabó L, et al. Targeting vascular endothelial growth factor receptor 2 and protein kinase D1 related pathways by a multiple kinase inhibitor in angiogenesis and inflammation related processes in vitro. PLoS One. 2015; 10:e0124234.

24. Chen H, Cong Q, Du Z, Liao W, Zhang L, Yao Y, et al. Sulfated fucoidan FP08S2 inhibits lung cancer cell growth in vivo by disrupting angiogenesis via targeting VEGFR2/VEGF and blocking VEGFR2/Erk/VEGF signaling. Cancer Lett. 2016; 382:44–52.

25. DeWire M, Fouladi M, Turner DC, Wetmore C, Hawkins C, Jacobs C, et al. An open-label, two-stage, phase II study of bevacizumab and lapatinib in children with recurrent or refractory ependymoma: a collaborative ependymoma research network study (CERN). J Neurooncol. 2015; 123:85–91.

26. Liu H, Wu B, Pan G, He L, Li Z, Fan M, et al. Metabolism and pharmacokinetics of mangiferin in conventional rats, pseudo-germ-free rats, and streptozotocin-induced diabetic rats. Drug Metab Dispos. 2012; 40:2109–2118.

27. Zhao J, Zhang B, Li S, Zeng L, Chen Y, Fang J. Mangiferin increases Nrf2 protein stability by inhibiting its ubiquitination and degradation in human HL60 myeloid leukemia cells. Int J Mol Med. 2014; 33:1348–1354.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download