Abstract
Purpose
Gonadotropin-releasing hormone (GnRH) agonists have been used with adjuvant chemotherapy to protect ovarian function. However, there are no data on the actual pregnancy rates among young breast cancer patients receiving GnRH agonists and concurrent chemotherapy in Korea.
Methods
Among patients who underwent surgery from January 2002 to April 2012, premenopausal patients aged between 20 and 40 years were included in the analysis. We retrospectively reviewed clinicopathologic features (e.g., age, obstetric and menstruation history), recurrence, and survival status. The rate of resumption of menstruation was calculated in all patients. In the married group, pregnancy and delivery rates were also recorded.
Results
Among 101 patients, 19 were lost to follow-up and 82 were eligible for the analysis. Among them, 31 were married, 10 of 51 got married, and 41 remained unmarried through the follow-up period. Among the married patients, 15 became pregnant and gave birth to 19 babies, whereas 26 did not become pregnant. The pregnancy rate in the married group was 50.0% (15/30). Three of 15 pregnancies (20.0%) were multiparous. Most of the delivered babies were healthy and 80.0% of patients had no problems breastfeeding (12/15). More than half the patients in all groups recovered menstrual status within 12 months.
Breast cancer is the most common cancer of women in the world. In Korea, 17,792 patients were diagnosed as having breast cancer during 2012; 46.6% of these patients were premenopausal [123].
One of the major concerns for young female breast cancer patients is whether the ability for menstruation and pregnancy will be terminated due to the disease. The results of a survey “Helping Ourselves, Helping Others: The Young Women's Breast Cancer Study” revealed that over 50% of women younger than 40 years of age, upon diagnosis, are concerned about future fertility, and a significant proportion wish to consider pregnancy after breast cancer treatment [45].
Gonadotropin-releasing hormone (GnRH) agonists have been used with adjuvant chemotherapy to protect ovarian function [6]. Trials of the GnRH agonist functionality have shown mixed results. In the GBG 37 ZORO study, premenopausal patients with breast cancer who received goserelin simultaneously with modern neoadjuvant chemotherapy did not experience statistically significant lower rates of amenorrhea after the end of chemotherapy compared with those receiving chemotherapy alone [7]. In contrast, in the POEMS/S0230 trial, the administration of goserelin along with chemotherapy reduced the risk of early menopause and improved the prospects of fertility [8].
Moreover, there are no data about the actual pregnancy rates among young breast cancer patients receiving GnRH agonists and concurrent chemotherapy in Korea. Therefore, we retrospectively evaluated the rates of pregnancy and resumption of menstruation in young Korean breast cancer patients.
We retrospectively reviewed the medical records of patients with invasive breast cancer who underwent surgery at the Breast Cancer Center of Samsung Medical Center, Seoul, Korea and conducted telephone surveys to obtain additional data. Patients who underwent surgery from January 2002 to April 2012 were eligible for this study because GnRH agonists for ovarian protection were used in extensively after 2002 at this institution. All patients decided to use GnRH agonists themselves after getting information about GnRH agonists in the outpatient department. Among them, premenopausal patients aged between 20 and 40 years were included in the analysis. All patients were treated with adjuvant chemotherapy, although the chemotherapy regimens were not all the same. Women with estrogen receptor (ER)-positive tumors were offered tamoxifen for 5 years, and those treated with breast conserving surgeries were offered adjuvant radiotherapy after the end of chemotherapy. Patients were excluded if they had distant metastasis at diagnosis or a history of ipsilateral or contralateral breast cancer, received neoadjuvant chemotherapy, or previously undergone bilateral salpingo-oophorectomy for any reason, or if they had lost contact with clinicians immediately after surgery. Patients were also excluded if they used GnRH agonists sequentially after chemotherapy for adjuvant therapy, as alternative endocrine treatment after recurrence, or for the treatment of conditions other than breast cancer, such as endometriosis.
During this period, GnRH agonists were administered to 101 patients via subcutaneous injection 2 weeks before the initiation of chemotherapy and administered every 28 days until the completion of chemotherapy. Two kinds of GnRH agonists were used in the study: leuprorelin 3.75 mg (Leuplin®; Takeda Chemical Industries Ltd., Osaka, Japan) for 60 women and goserelin 3.6 mg (ZOLADEX®; AstraZeneca Pharma International, Cambridge, UK) for 41 women.
We retrospectively reviewed clinicopathologic features, including pathological stage, nuclear grade, histological grade, immunohistochemical findings (e.g., ER, progesterone receptor, and human epidermal growth factor receptor 2 status), treatment modalities, and recurrence or survival status. In addition, age and obstetric and menstruation history were included. In the married group, pregnancy and delivery rate were calculated. In the unmarried group, the rate of resumption of menstruation was calculated. Median follow-up duration was 7.48 years (range, 4–14 years). This study was approved by the Institutional Review Board of Samsung Medical Center (IRB number: 2016-03-123-0001).
All data were analyzed using IBM SPSS Statistics version 23.0 software (IBM Corp., Armonk, USA). Chi-square tests or linear by linear associations were used to compare baseline clinicopathologic details between the pregnancy and nonpregnancy groups. One way analysis of variance, was used to check differences in menstruation recovery in each group and Spearman rank correlation analysis was used to reveal the interrelationship between resumption of menstruation and age at diagnosis or menarche. A p-value <0.05 was considered statistically significant.
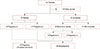
Out of 101 patients, 19 were lost to follow-up and 82 were eligible for inclusion in the study. Among these patients, 31 patients were in the married group, 10 of 51 patients initially in the unmarried group got married during follow-up, and 41 patients remained unmarried throughout the follow-up period. Among the married group, a total of 15 patients became pregnant and gave birth to 19 babies, whereas 26 patients did not become pregnant (Figure 1).
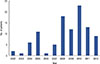
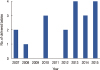
In the initial period, only 1 to 2 cases were treated with GnRH agonists annually. After 2008, the use of GnRH agonists increased, but the increase was not linear over time. The greatest annual use of GnRH agonists was 23 cases in 2010 (Figure 2). The first delivery in the married group was more than 5 years after the initial use of GnRH agonists. After 2012, the number of delivered babies was consistently greater than two per year (Figure 3). Table 1 shows the number of patients in the married group who achieved pregnancy and breastfeeding. Among the 41 patients in the ever-married group, 15 became pregnant and gave birth to one or more babies. Among the 26 patients who did not become pregnant, 11 did not attempt pregnancy and 15 did not become pregnant despite attempts. There was one case of a delayed pregnancy attempt for additional chemotherapy; this patient was 21 years old at diagnosis and had stage IIA disease. At 1 year after partial mastectomy, she underwent (video-assisted thoracoscopic surgery) metastasectomy for lung metastasis and gave up trying for pregnancy to receive additional chemotherapy. Two of the 15 patients who unsuccessfully attempted pregnancy tried in vitro fertilization. All patients who achieved pregnancy did so through a sexual relationship. The pregnancy rate among women who attempted pregnancy was 50.0% (15/30). Three of 15 pregnancies were multiparous (20.0%). In most cases, the delivered babies were healthy and no adverse events occurred during pregnancy and delivery. There was one case of a preterm infant who needed neonatal intensive care unit care. Most patients who delivered babies had no problems breastfeeding (12/15, 80.0%). Among the 12 patients who successfully breastfed, seven received breast conserving surgery and five underwent total mastectomy. All patients used the contralateral breast for breastfeeding. The breastfeeding duration was 1 to 12 months. Table 2 shows the duration from each treatment to pregnancy in 15 pregnant patients. Achieving pregnancy took more than 40 months from the last chemotherapy session and surgery. The mean duration from last tamoxifen dosage to pregnancy was 18 months, which occurred in only six ER-positive patients.
The clinicopathologic characteristics of patients in the pregnancy and nonpregnancy groups are listed in Table 3. Median age was similar in the pregnancy group (range, 22–39 years) and nonpregnancy group (range, 21–40 years). Most of the patients in both groups were stage II. The rate of ER-negative cancer tended to be higher in the pregnancy group than in the nonpregnancy group, and the rate of ER positivity tended to be higher in the nonpregnancy group, but there was no statistically significant difference. Among the six patients in the pregnancy group who were treated with tamoxifen, five patients received tamoxifen for 5 years and one patient for 1 year. Among the 15 patients treated with tamoxifen in the nonpregnancy group, 14 took tamoxifen for 5 years and one patient took it for 1 year. FAC (fluorouracil+doxorubicin+cycl ophosphamide) was the most frequently used regimen in the pregnancy group, but all regimens were evenly used in the nonpregnancy group. There was no correlation between the chemotherapy regimen and pregnancy. Disease-free survival time was similar in both groups.
More than half of patients restarted menstruation within 1year in all groups (Figure 4). In the nonpregnancy and still unmarried groups, only one patient in each group did not resume menstruation. The duration from end of chemotherapy to menstruation in each group did not differ statistically (Table 4). Most of 40 patients in the still unmarried group restarted menstruation within 12 months regardless of age and menarche (Table 5).
In our survey, the pregnancy rate in young Korean breast cancer patients treated with concurrent chemotherapy and GnRH agonists was 50.0% (15/30), and 20.0% of the pregnancies were multiparous (3/15). Most of the delivered babies were healthy and 80.0% had no problems breastfeeding (12/15). More than half of the patients in all groups recovered menstruation within 12 months.
Several previous studies report the pregnancy rate in similar conditions. Shen et al. [9] showed comparable spontaneous pregnancy rates in patients who received chemotherapy with and without GnRH agonist use (odds ratio [OR], 0.177; 95% confidence interval [CI], 0.92–1.40; p=0.09). In contrast, in the POEMS/S0230 trial, the pregnancy rate was 11% in the chemotherapy alone group and 21% in the goserelin group (OR, 2.45; 95% CI, 1.09–5.51; p=0.03) [8]. A meta-analysis by Munhoz et al. [10] also revealed a higher number of pregnancies in the GnRH group than in the chemotherapy alone group (OR, 1.85; 95% CI, 1.02–3.36; p=0.04). The fertility rate in our study was higher than that in other studies. This is probably due to the small number of patients and the analysis of only married patients who attempted pregnancy. Another study of pregnancy rates in patients who attempted pregnancy showed a 71% pregnancy rate [11]. In our study population, the pregnancy rate in all married patients regardless of attempt was 36.5%. Meanwhile, our study retrospectively selected patients who used GnRH agonists, and these patients not only decided to use of GnRH agonists themselves, but also purchased it. GnRH agonists are quite expensive, and some patients who may have desired pregnancy could not use it due to financial constraints. Thus, it is possible that the pregnancy rate observed in our study was influenced by selection bias.
Several studies have reported similar numbers of reported miscarriages, elective terminations, and pregnancy complications, irrespective of GnRH agonist use [810]. Wong et al. [11] reported that, among 30 patients who achieved pregnancy under GnRH agonist use with chemotherapy, there were five spontaneous miscarriages, three voluntary terminations for social reasons, and one case of ectopic pregnancy. In previous reports, the rate of congenital abnormalities of infants born to women with a history of breast cancer ranges was 0% to 7.2%. Considering that the percentage of congenital abnormalities in the general population is nearly 4%, the rate observed in these women is similar to that of the general population [12]. Therefore, patients do not need to abandon pregnancy after breast cancer for fear of adverse events.
There were no differences in clinicopathologic characteristics between the pregnancy and nonpregnancy groups in our study. ER-negative status was more common in the pregnancy group than the nonpregnancy group, but the difference was not significant (p=0.133). In a multicenter retrospective study including 333 pregnancies after breast cancer and 874 matched nonpregnant controls, ER status did not influence pregnancy [13]. The main adjuvant endocrine therapy for premenopausal patients with receptor-positive breast cancer is a 5-year course of tamoxifen. During pregnancy, tamoxifen and its metabolites interact with rapidly growing and developing embryonic or fetal tissues. The relatively high frequency of severe congenital abnormalities indicates that reliable birth control is mandatory during tamoxifen treatment. After tamoxifen use, a 2-month washout period is advisable based on the known half-life of tamoxifen [14]. In our study, patients in the pregnancy group had a greater chance of becoming pregnant because the rate of tamoxifen use was lower than in the nonpregnancy group. Moreover, the “healthy mom effect” noted in previous studies proposes that only women who feel healthy give birth and those who are affected by their disease do not. Thus, the women who become pregnant after breast cancer treatment may represent a group of women who are free of relapse and/or generally healthier than women in the nonpregnant group [1516]. If this notion could be applied to our results, disease-free survival is higher in the pregnancy group than in the nonpregnancy group. However, we could not find a statistical intergroup difference. We found that disease-free survival tended to be higher in the pregnancy group than in the nonpregnancy group. This could be due to our small study population.
In previous reports, menstruation recovery was faster and more common in the GnRH agonist group than in the chemotherapy alone group [79]. Another study described the relationship between menstruation resumption and age, showing that the incidence of anticancer treatment related ovarian failure increased with increasing age in 22% to 61% and 61% to 97% of women aged <40 years and >40 years, respectively [17]. The high resumption rate of menstruation in our study could not be explained by the effect of GnRH agonist because our study had no control group without GnRH agonist use. However, it could be explained by young age because only premenopausal patients aged 20 to 40 years were included in our study.
We note that our study had several limitations. First, the small sample size and loss of 19 patients in follow-up limited our power to calculate the fertility rate. Second, the absence of a control group prevented an analysis of clinicopathologic factors. Third, there were no data on hormones such as follicle-stimulating hormone, anti-Mullerian hormone, luteinizing hormone, estradiol (E2), and inhibin A or B, which help detect chemotherapy-induced ovarian damage prior to menstruation status. Despite these limitations, this study is valuable in that it revealed the real pregnancy rate of Korean young breast cancer patients using GnRH agonists with chemotherapy. Moreover, the use of additional telephone surveys improved the reliability of our results.
Although GnRH agonists have potential side effects including hot flashes, headaches, mood changes, sweating, and dry skin [18], it is used by young breast cancer patients in many countries who wish to retain the option of pregnancy. Several studies recently reported other advantages of GnRH agonists. The concurrent administration of GnRH agonists during neoadjuvant chemotherapy improved pathologic complete response rates and suppressed Ki-67 expression, especially in hormone receptor negative tumors [19], and showed better recurrence-free survival than treatment with chemotherapy alone (adjusted hazard ratio, 0.21; p=0.009; unadjusted hazard ratio, 0.33; p=0.034) [20].
We surveyed the real pregnancy rate in Korean young breast cancer patients treated with concurrent chemotherapy and GnRH agonists. A further study with a control group is required to confirm whether the use of GnRH agonists improves pregnancy rates.
Figures and Tables
 | Figure 4Menstruation resumption. (A) In pregnancy group, (B) in nonpregnancy group, and (C) in still unmarried group. X-axis means each patient in due order from with the shortest recovery months to the longest recovery months. Y-axis means interval months to menstruation resumption in each patient. The dotted line indicates range of menstrual recovery within 1 year. |
Table 1
Pregnancy outcomes

Table 2
Duration from each treatment to pregnancy in 15 pregnant patients

Table 3
Characteristics of 41 patients in married status

Table 4
Duration from end of chemotherapy to menstruation

| Group | Duration (mo), mean ± SD (range) | p-value |
|---|---|---|
| Pregnancy | 16.47 ± 10.7 (2–63) | 0.720 |
| Nonpregnancy | 11.77 ± 7.66 (1–67) | |
| Still unmarried | 13.25 ± 7.71 (1–77) |
Table 5
Interval months to resumption of menstruation in unmarried patients

Notes
References
1. Jung KW, Won YJ, Kong HJ, Oh CM, Cho H, Lee DH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015; 47:127–141.

2. American Cancer Society. Breast cancer facts & figures 2015-2016. March 1st, 2016. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-046381.pdf.
3. Assi HA, Khoury KE, Dbouk H, Khalil LE, Mouhieddine TH, El Saghir NS. Epidemiology and prognosis of breast cancer in young women. J Thorac Dis. 2013; 5:Suppl 1. S2–S8.
4. Pagani O, Partridge A, Korde L, Badve S, Bartlett J, Albain K, et al. Pregnancy after breast cancer: if you wish, ma'am. Breast Cancer Res Treat. 2011; 129:309–317.

5. Anders CK, Johnson R, Litton J, Phillips M, Bleyer A. Breast cancer before age 40 years. Semin Oncol. 2009; 36:237–249.

6. Bedoschi G, Turan V, Oktay K. Utility of GnRH-agonists for fertility preservation in women with operable breast cancer: is it protective? Curr Breast Cancer Rep. 2013; 5:302–308.

7. Gerber B, von Minckwitz G, Stehle H, Reimer T, Felberbaum R, Maass N, et al. Effect of luteinizing hormone-releasing hormone agonist on ovarian function after modern adjuvant breast cancer chemotherapy: the GBG 37 ZORO study. J Clin Oncol. 2011; 29:2334–2341.

8. Moore HC, Unger JM, Phillips KA, Boyle F, Hitre E, Porter D, et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med. 2015; 372:923–932.

9. Shen YW, Zhang XM, Lv M, Chen L, Qin TJ, Wang F, et al. Utility of gonadotropin-releasing hormone agonists for prevention of chemotherapy-induced ovarian damage in premenopausal women with breast cancer: a systematic review and meta-analysis. Onco Targets Ther. 2015; 8:3349–3359.

10. Munhoz RR, Pereira AA, Sasse AD, Hoff PM, Traina TA, Hudis CA, et al. Gonadotropin-releasing hormone agonists for ovarian function preservation in premenopausal women undergoing chemotherapy for early-stage breast cancer: a systematic review and meta-analysis. JAMA Oncol. 2016; 2:65–73.

11. Wong M, O'Neill S, Walsh G, Smith IE. Goserelin with chemotherapy to preserve ovarian function in pre-menopausal women with early breast cancer: menstruation and pregnancy outcomes. Ann Oncol. 2013; 24:133–138.

12. Langagergaard V, Gislum M, Skriver MV, Nørgård B, Lash TL, Rothman KJ, et al. Birth outcome in women with breast cancer. Br J Cancer. 2006; 94:142–146.

13. Azim HA Jr, Kroman N, Paesmans M, Gelber S, Rotmensz N, Ameye L, et al. Prognostic impact of pregnancy after breast cancer according to estrogen receptor status: a multicenter retrospective study. J Clin Oncol. 2013; 31:73–79.

14. Braems G, Denys H, De Wever O, Cocquyt V, Van den Broecke R. Use of tamoxifen before and during pregnancy. Oncologist. 2011; 16:1547–1551.

15. Sankila R, Heinävaara S, Hakulinen T. Survival of breast cancer patients after subsequent term pregnancy: “healthy mother effect”. Am J Obstet Gynecol. 1994; 170:818–823.

16. Gelber S, Coates AS, Goldhirsch A, Castiglione-Gertsch M, Marini G, Lindtner J, et al. Effect of pregnancy on overall survival after the diagnosis of early-stage breast cancer. J Clin Oncol. 2001; 19:1671–1675.

17. Lambertini M, Anserini P, Levaggi A, Poggio F, Del Mastro L. Fertility counseling of young breast cancer patients. J Thorac Dis. 2013; 5:Suppl 1. S68–S80.
18. Yang B, Shi W, Yang J, Liu H, Zhao H, Li X, et al. Concurrent treatment with gonadotropin-releasing hormone agonists for chemotherapy-induced ovarian damage in premenopausal women with breast cancer: a meta-analysis of randomized controlled trials. Breast. 2013; 22:150–157.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download