Abstract
Two consecutive surveys for breast surgeons in Korea were conducted to comprehend the practice patterns and perceptions on margin status after breast-conserving surgery. The surveys were conducted online in 2014 (initial) and 2016 (follow-up). A total of 126 and 88 responses were obtained in the initial and follow-up survey, respectively. More than 80% of the respondents replied to routinely apply frozen section biopsy for intraoperative margin assessment in both surveys. Re-excision recommendations of the margin for invasive cancer significantly changed from a close margin to a positive margin over time (p=0.033). Most of the respondents (73.8%) defined a negative margin as “no ink on tumor” in invasive cancer, whereas more diverse responses were observed in ductal carcinoma in situ cases. The influence of guideline establishment for negative margins has been identified. A high uptake rate of intraoperative frozen section biopsy was noted and routine use needs reconsideration.
The definition of a negative margin was recently established for patients undergoing breast-conserving surgery (BCS) with whole-breast irradiation by the Society of Surgical Oncology (SSO), American Society for Radiation Oncology (ASTRO), and American Society of Clinical Oncology [12]. Negative margin is one of the strongest prognostic factors for ipsilateral breast tumor recurrence, and to obtain a negative margin, reexcision is frequently performed [3]. In effort to reduce reoperation rates, a demand for tools to assess the margin intraoperatively, such as specimen mammography, intraoperative ultrasound, frozen section and cytology has been generated. However, none of these techniques have been adopted universally due to their varied accuracy and cost-effectiveness [4].
We have conducted a survey on these issues to comprehend the practice patterns of breast surgeons in Korea. We sent consecutive surveys via e-mail to the members of the Korean Breast Cancer Society in April 2014 (initial survey) and November 2016 (follow-up survey). Only breast surgeons who currently perform breast cancer surgeries were asked to respond. Ten questions regarding the intraoperative evaluation of the margin and decision on whether re-excision should be performed were evaluated. In the follow-up survey, three questions were added about the definition of negative margin and intraoperative gross target margin. We analyzed perceptions of negative resection margins, intraoperative margin assessment methods, and how perceptions and methods change over time. Ethical approval was obtained from the Catholic Medical Center Institutional Review Board (IRB number: KC16QISI0942). Results were analyzed using the chi-square test or Fisher exact test with SPSS version 24.0 (IBM Corp., Armonk, USA). Statistical significance was assumed at a p-value of <0.05.
A total of 126 breast surgeons from 79 institutions participated in the initial survey in April 2014, and 88 surgeons from 59 institutions participated in the follow-up survey in November 2016. Among them, 63 respondents participated in both surveys. The composition of respondents regarding the year of specialist certification in general surgery did not differ between the two surveys (p=0.723). One-eighth of respondents became specialists between 1978 and 1989, one-fourth between 1990 and 1999, and the rest between 2000 and 2015.
Most respondents in both surveys responded that they “always” perform an intraoperative biopsy to assess the margin (81.7% in the initial and 84.1% in the follow-up survey) (Figure 1). Approximately 10% of respondents in both surveys answered that they perform it only when margins were unclear on gross examination. While, 7.9% and 3.4% respondents from the initial and follow-up survey, respectively, replied that they never performed an intraoperative biopsy.
All surgeons who performed intraoperative pathologic margin evaluation responded to use frozen section biopsy. The surgical technique used for margin evaluation was substantially heterogeneous. The responds from the initial and follow-up surveys were as follows, respectively: (1) obtaining separate breast tissue samples from the cavity: 44.8%, 50.6%; (2) obtaining separate breast tissue samples from the specimen: 44.0%, 36.5%; (3) shaved margin sampling or total cavity circumference excisions: 11.2%, 10.5%; (4) obtaining separate breast tissue samples from the cavity or specimen according to the specimen's gross margin: 0%, 2.3%.
When asked whether intraoperative conversion to total mastectomy was performed according to frozen section biopsy results, 58.8% and 53.4% from the initial and follow-up survey replied that they only change surgical plans when discussed with the patient preoperatively. However, more than one-third of respondents (37.1% at initial, 34.1% at follow-up survey) changed surgical plans regardless of preoperative discussion with the patient. Additionally, at the follow-up survey, one surgeon (1.2%) commented that he or she converts to total mastectomy after discussing with family members during surgery. Surgeons who do not perform intraoperative frozen section biopsy, replied that they do not convert to total mastectomy.
The surgeons' recommendations for re-excision of the margin are shown in Table 1 according to invasive cancer and ductal carcinoma in situ (DCIS). Table 1 also compares the results of both surveys. Respondents were asked to choose their reply based on their institution's pathology report (numeric margin: positive, margin ≤1 mm, ≤2 mm, ≤5 mm, and ≤10 mm; rough margin: positive, very close, close margin). For invasive cancer, respondents significantly changed their re-excision recommendation from a close margin to a positive margin at the follow-up survey (p=0.033). In cases of DCIS, a non-significant change to a narrower indication for re-excision was noticed in the follow-up survey. Furthermore, the proportion of respondents who do not recommend performing re-excision reduced in the follow-up survey. Similar results were shown when answers were analyzed only among surgeons who completed both surveys (data not shown).
At the follow-up survey in November 2016, the definitions of negative margins for invasive cancer and DCIS were additionally asked (Figure 2). For invasive cancer, 73.8% of respondents defined a negative margin as “no ink on tumor.” The definition of a negative margin in DCIS was more variable. The response rate of “no ink on tumor” for a negative margin was 48.9%, while 19.3% defined it as more than 1 mm from the margin and 20.5% as more than 2 mm. The definition of a negative margin was significantly wider for DCIS compared to invasive cancer (Fisher exact test, p=0.005).
The SSO-ASTRO guidelines for margin status were published between the two surveys. In this study, respondents' replies significantly changed with respect to consideration of reexcision in invasive cases for only a positive margin, not a close margin, reflecting the influence of the establishment of the aforementioned guidelines. To our knowledge, this is the first study to directly compare responses over time. However, when comparing results from survey studies performed before and after guideline publication, more surgeons responded that they perform re-excision only for positive margins after guideline publication [567]. However, when complex scenarios are suggested, the response variation was wider [5]. We did not suggest any scenarios in our study, but several surgeons did mention to apply different indications in different circumstances. The SSO-ASTRO guidelines are built upon weak evidence, and individual judgment and flexibility are needed when applying these guidelines clinically [12]. More clinical trials that reflect various situations are needed to provide stronger evidence.
Considering the uptake rates of 0% to 18% from North America and Europe, the fact that more than 80% of surgeons involved in this study perform frozen section biopsy for intraoperative margin evaluation is remarkable [468]. Although frozen section biopsy is one of the most accurate tool for intraoperative margin assessment, its uptake rate is generally poor [4]. Frozen section biopsy is a time and cost-consuming method that disrupts surgical workflow and is only routinely available in high-volume centers with large pathology teams [4]. However, in Korea, the cost is relatively low and accessibility is relatively high, as more than 80% of cancer surgeries are performed in high-volume centers [9].
The high uptake of frozen section biopsy in Korea is also worrisome as the cost-effectiveness and efficacy in reducing re-excision rate have not been demonstrated properly. Current publications are all based on retrospective studies from small centers [1011]. Clinical guidelines also do not require a frozen section for optimal evaluation of the margin [12]. Despite the low level of evidence for this technique, most Korean surgeons apply it routinely due to surgical training and concerns about re-operation. Consideration of the current evidence is needed, and efforts to generate more evidence must be executed.
This study is the first to report on Korean surgeons' practice patterns regarding BCS. Although response bias can occur due to low response rates and self-reporting, it was a national survey and more than 80% of academic centers in Korea participated. The variations among surgeons regarding definition of a negative margin and indication for re-excision were revealed in this study. A change of indications for re-excision in invasive cancer was noticed, reflecting the influence of guidelines on clinical practice. The substantially high uptake rate of intraoperative frozen section biopsy for margin evaluation must also be noted. Routine use of frozen section biopsy must be reconsidered, and clinical trials are needed to build evidence.
Figures and Tables
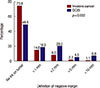 | Figure 2Definition of negative margin in breast-conserving surgery (follow-up survey only).DCIS=ductal carcinoma in situ.
|
Table 1
Decision for re-excision of margin according to type of cancer and resection margin distance

ACKNOWLEDGMENTS
We are sincerely grateful to the many members of the Korean Breast Cancer Society who participated in the two surveys of this study.
References
1. Buchholz TA, Somerfield MR, Griggs JJ, El-Eid S, Hammond ME, Lyman GH, et al. Margins for breast-conserving surgery with whole-breast irradiation in stage I and II invasive breast cancer: American Society of Clinical Oncology endorsement of the Society of Surgical Oncology/American Society for Radiation Oncology consensus guideline. J Clin Oncol. 2014; 32:1502–1506.

2. Morrow M, Van Zee KJ, Solin LJ, Houssami N, Chavez-MacGregor M, Harris JR, et al. Society of Surgical Oncology-American Society for Radiation Oncology-American Society of Clinical Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. J Clin Oncol. 2016; 34:4040–4046.

3. Meric F, Mirza NQ, Vlastos G, Buchholz TA, Kuerer HM, Babiera GV, et al. Positive surgical margins and ipsilateral breast tumor recurrence predict disease-specific survival after breast-conserving therapy. Cancer. 2003; 97:926–933.

4. St John ER, Al-Khudairi R, Ashrafian H, Athanasiou T, Takats Z, Hadjiminas DJ, et al. Diagnostic accuracy of intraoperative techniques for margin assessment in breast cancer surgery: a meta-analysis. Ann Surg. 2017; 265:300–310.

5. DeSnyder SM, Hunt KK, Smith BD, Moran MS, Klimberg S, Lucci A. Assessment of practice patterns following publication of the SSO-ASTRO consensus guideline on margins for breast-conserving therapy in stage I and II invasive breast cancer. Ann Surg Oncol. 2015; 22:3250–3256.

6. Parvez E, Hodgson N, Cornacchi SD, Ramsaroop A, Gordon M, Farrokhyar F, et al. Survey of American and Canadian general surgeons’ perceptions of margin status and practice patterns for breast conserving surgery. Breast J. 2014; 20:481–488.

7. Taghian A, Mohiuddin M, Jagsi R, Goldberg S, Ceilley E, Powell S. Current perceptions regarding surgical margin status after breast-conserving therapy: results of a survey. Ann Surg. 2005; 241:629–639.

8. Blair SL, Thompson K, Rococco J, Malcarne V, Beitsch PD, Ollila DW. Attaining negative margins in breast-conservation operations: is there a consensus among breast surgeons? J Am Coll Surg. 2009; 209:608–613.

9. Yun YH, Kim YA, Min YH, Park S, Won YJ, Kim DY, et al. The influence of hospital volume and surgical treatment delay on long-term survival after cancer surgery. Ann Oncol. 2012; 23:2731–2737.

10. Boughey JC, Hieken TJ, Jakub JW, Degnim AC, Grant CS, Farley DR, et al. Impact of analysis of frozen-section margin on reoperation rates in women undergoing lumpectomy for breast cancer: evaluation of the National Surgical Quality Improvement Program data. Surgery. 2014; 156:190–197.

11. Jorns JM, Visscher D, Sabel M, Breslin T, Healy P, Daignaut S, et al. Intraoperative frozen section analysis of margins in breast conserving surgery significantly decreases reoperative rates: one-year experience at an ambulatory surgical center. Am J Clin Pathol. 2012; 138:657–669.

12. National Comprehensive Cancer Network. Clinical practice guidelines in oncology: breast cancer. Accessed May 4th, 2017. https://www.nccn.org/professionals/physician_gls/default.aspx.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download