1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90. PMID:
21296855.

2. Dixon JM, Page DL, Anderson TJ, Lee D, Elton RA, Stewart HJ, et al. Long-term survivors after breast cancer. Br J Surg. 1985; 72:445–448. PMID:
4016513.

3. Jezierska A, Motyl T. Matrix metalloproteinase-2 involvement in breast cancer progression: a mini-review. Med Sci Monit. 2009; 15:RA32–RA40. PMID:
19182722.
4. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, et al. Strategies for subtypes: dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011; 22:1736–1747. PMID:
21709140.
5. Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004; 20:781–810. PMID:
15473860.

6. Kirikoshi H, Katoh M. Expression of WNT7A in human normal tissues and cancer, and regulation of WNT7A and WNT7B in human cancer. Int J Oncol. 2002; 21:895–900. PMID:
12239632.

7. Avgustinova A, Iravani M, Robertson D, Fearns A, Gao Q, Klingbeil P, et al. Tumour cell-derived Wnt7a recruits and activates fibroblasts to promote tumour aggressiveness. Nat Commun. 2016; 7:10305. PMID:
26777421.

8. Liu Y, Meng F, Xu Y, Yang S, Xiao M, Chen X, et al. Overexpression of Wnt7a is associated with tumor progression and unfavorable prognosis in endometrial cancer. Int J Gynecol Cancer. 2013; 23:304–311. PMID:
23321718.

9. Zhang XL, Peng CJ, Peng J, Jiang LY, Ning XM, Zheng JH. Prognostic role of Wnt7a expression in ovarian carcinoma patients. Neoplasma. 2010; 57:545–551. PMID:
20845993.

10. Ramos-Solano M, Meza-Canales ID, Torres-Reyes LA, Alvarez-Zavala M, Alvarado-Ruíz L, Rincon-Orozco B, et al. Expression of WNT genes in cervical cancer-derived cells: implication of WNT7A in cell proliferation and migration. Exp Cell Res. 2015; 335:39–50. PMID:
25978974.

11. Bikkavilli RK, Avasarala S, Van Scoyk M, Arcaroli J, Brzezinski C, Zhang W, et al. Wnt7a is a novel inducer of beta-catenin-independent tumor-suppressive cellular senescence in lung cancer. Oncogene. 2015; 34:5317–5328. PMID:
25728679.
12. Ochoa-Hernández AB, Ramos-Solano M, Meza-Canales ID, García-Castro B, Rosales-Reynoso MA, Rosales-Aviña JA, et al. Peripheral T-lymphocytes express WNT7A and its restoration in leukemia-derived lymphoblasts inhibits cell proliferation. BMC Cancer. 2012; 12:60. PMID:
22313908.

13. Kondratov AG, Kvasha SM, Stoliar LA, Romanenko AM, Zgonnyk YM, Gordiyuk VV, et al. Alterations of the WNT7A gene in clear cell renal cell carcinomas. PLoS One. 2012; 7:e47012. PMID:
23056560.

14. Hirata T, Zheng Q, Chen Z, Kinoshita H, Okamoto J, Kratz J, et al. Wnt7A is a putative prognostic and chemosensitivity marker in human malignant pleural mesothelioma. Oncol Rep. 2015; 33:2052–2060. PMID:
25632963.

15. Peng C, Zhang X, Wang Y, Li L, Wang Q, Zheng J. Expression and prognostic significance of Wnt7a in human endometrial carcinoma. Obstet Gynecol Int. 2012; 2012:134962. PMID:
23304155.

16. Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987; 8:138–140. PMID:
3303008.
17. Ohira T, Gemmill RM, Ferguson K, Kusy S, Roche J, Brambilla E, et al. WNT7a induces E-cadherin in lung cancer cells. Proc Natl Acad Sci U S A. 2003; 100:10429–10434. PMID:
12937339.

18. Yoshioka S, King ML, Ran S, Okuda H, MacLean JA 2nd, McAsey ME, et al. WNT7A regulates tumor growth and progression in ovarian cancer through the WNT/beta-catenin pathway. Mol Cancer Res. 2012; 10:469–482. PMID:
22232518.
19. Hirabayashi Y, Itoh Y, Tabata H, Nakajima K, Akiyama T, Masuyama N, et al. The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 2004; 131:2791–2801. PMID:
15142975.
20. Lucas FR, Salinas PC. WNT-7a induces axonal remodeling and increases synapsin I levels in cerebellar neurons. Dev Biol. 1997; 192:31–44. PMID:
9405095.

21. Lyu J, Joo CK. Wnt-7a up-regulates matrix metalloproteinase-12 expression and promotes cell proliferation in corneal epithelial cells during wound healing. J Biol Chem. 2005; 280:21653–21660. PMID:
15802269.

22. Winn RA, Marek L, Han SY, Rodriguez K, Rodriguez N, Hammond M, et al. Restoration of Wnt-7a expression reverses non-small cell lung cancer cellular transformation through frizzled-9-mediated growth inhibition and promotion of cell differentiation. J Biol Chem. 2005; 280:19625–19634. PMID:
15705594.

23. Liu Z, Wang L, Yang J, Bandyopadhyay A, Kaklamani V, Wang S, et al. Estrogen receptor alpha inhibits senescence-like phenotype and facilitates transformation induced by oncogenic Ras in human mammary epithelial cells. Oncotarget. 2016; 7:39097–39107. PMID:
27259243.

24. Choi M, Park YH, Ahn JS, Im YH, Nam SJ, Cho SY, et al. Evaluation of pathologic complete response in breast cancer patients treated with neoadjuvant chemotherapy: experience in a single institution over a 10-year period. J Pathol Transl Med. 2017; 51:69–78. PMID:
28013533.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


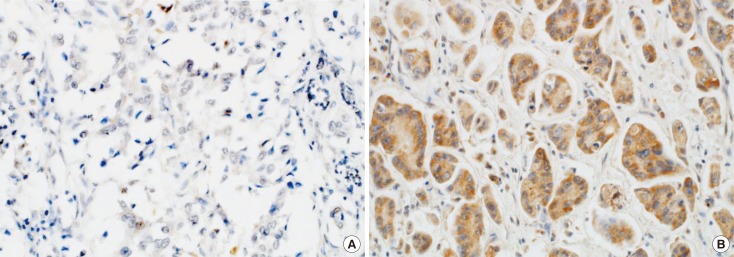
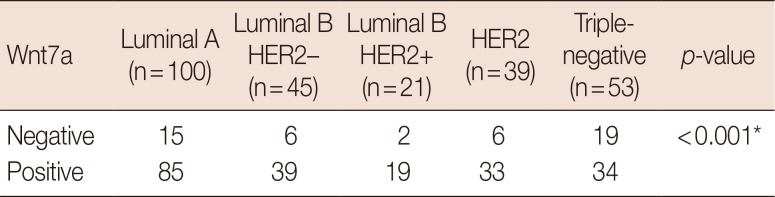
 XML Download
XML Download