1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108. PMID:
25651787.

2. Arriagada R, Spielmann M, Koscielny S, Le Chevalier T, Delozier T, Ducourtieux M, et al. Patterns of failure in a randomized trial of adjuvant chemotherapy in postmenopausal patients with early breast cancer treated with tamoxifen. Ann Oncol. 2002; 13:1378–1386. PMID:
12196363.

3. Blum JL, Jones SE, Buzdar AU, LoRusso PM, Kuter I, Vogel C, et al. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol. 1999; 17:485–493. PMID:
10080589.

4. Oshaughnessy JA, Blum J, Moiseyenko V, Jones SE, Miles D, Bell D, et al. Randomized, open-label, phase II trial of oral capecitabine (Xeloda) vs. a reference arm of intravenous CMF (cyclophosphamide, methotrexate and 5-fluorouracil) as first-line therapy for advanced/metastatic breast cancer. Ann Oncol. 2001; 12:1247–1254. PMID:
11697835.
5. Vahdat LT, Pruitt B, Fabian CJ, Rivera RR, Smith DA, Tan-Chiu E, et al. Phase II study of eribulin mesylate, a halichondrin B analog, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2009; 27:2954–2961. PMID:
19349550.

6. Livingston RB, Ellis GK, Gralow JR, Williams MA, White R, McGuirt C, et al. Dose-intensive vinorelbine with concurrent granulocyte colony-stimulating factor support in paclitaxel-refractory metastatic breast cancer. J Clin Oncol. 1997; 15:1395–1400. PMID:
9193331.

7. Smorenburg CH, Bontenbal M, Seynaeve C, van Zuylen C, de Heus G, Verweij J, et al. Phase II study of weekly gemcitabine in patients with metastatic breast cancer relapsing or failing both an anthracycline and a taxane. Breast Cancer Res Treat. 2001; 66:83–87. PMID:
11368414.

8. Thomas E, Tabernero J, Fornier M, Conté P, Fumoleau P, Lluch A, et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in patients with taxane-resistant metastatic breast cancer. J Clin Oncol. 2007; 25:3399–3406. PMID:
17606975.

9. Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005; 23:7794–7803. PMID:
16172456.

10. Gennari A, Conte P, Rosso R, Orlandini C, Bruzzi P. Survival of metastatic breast carcinoma patients over a 20-year period: a retrospective analysis based on individual patient data from six consecutive studies. Cancer. 2005; 104:1742–1750. PMID:
16149088.
11. Chia SK, Speers CH, D'yachkova Y, Kang A, Malfair-Taylor S, Barnett J, et al. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer. 2007; 110:973–979. PMID:
17647245.

12. Giordano SH, Buzdar AU, Smith TL, Kau SW, Yang Y, Hortobagyi GN. Is breast cancer survival improving? Cancer. 2004; 100:44–52. PMID:
14692023.

13. Mauri D, Polyzos NP, Salanti G, Pavlidis N, Ioannidis JP. Multiple-treatments meta-analysis of chemotherapy and targeted therapies in advanced breast cancer. J Natl Cancer Inst. 2008; 100:1780–1791. PMID:
19066278.

14. Fisher B, Brown AM, Dimitrov NV, Poisson R, Redmond C, Margolese RG, et al. Two months of doxorubicin-cyclophosphamide with and without interval reinduction therapy compared with 6 months of cyclophosphamide, methotrexate, and fluorouracil in positive-node breast cancer patients with tamoxifen-nonresponsive tumors: results from the National Surgical Adjuvant Breast and Bowel Project B-15. J Clin Oncol. 1990; 8:1483–1496. PMID:
2202791.

15. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005; 365:1687–1717. PMID:
15894097.
16. Bang SM, Heo DS, Lee KH, Byun JH, Chang HM, Noh DY, et al. Adjuvant doxorubicin and cyclophosphamide versus cyclophosphamide, methotrexate, and 5-fluorouracil chemotherapy in premenopausal women with axillary lymph node positive breast carcinoma. Cancer. 2000; 89:2521–2526. PMID:
11135211.

17. Early Breast Cancer Trialists' Collaborative Group. Polychemotherapy for early breast cancer: an overview of the randomized trials. Lancet. 1998; 352:930–942. PMID:
9752815.
18. Mamounas EP, Bryant J, Lembersky B, Fehrenbacher L, Sedlacek SM, Fisher B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol. 2005; 23:3686–3696. PMID:
15897552.

19. Jones S, Holmes FA, O'Shaughnessy J, Blum JL, Vukelja SJ, McIntyre KJ, et al. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of us oncology research trial 9735. J Clin Oncol. 2009; 27:1177–1183. PMID:
19204201.

20. Carmichael J, Possinger K, Phillip P, Beykirch M, Kerr H, Walling J, et al. Advanced breast cancer: a phase II trial with gemcitabine. J Clin Oncol. 1995; 13:2731–2736. PMID:
7595731.

21. Rha SY, Moon YH, Jeung HC, Kim YT, Sohn JH, Yang WI, et al. Gemcitabine monotherapy as salvage chemotherapy in heavily pretreated metastatic breast cancer. Breast Cancer Res Treat. 2005; 90:215–221. PMID:
15830134.

22. Vogel C, O'Rourke M, Winer E, Hochster H, Chang A, Adamkiewicz B, et al. Vinorelbine as first-line chemotherapy for advanced breast cancer in women 60 years of age or older. Ann Oncol. 1999; 10:397–402. PMID:
10370781.

23. Blum JL, Dieras V, Lo Russo PM, Horton J, Rutman O, Buzdar A, et al. Multicenter, phase II study of capecitabine in taxane-pretreated metastatic breast carcinoma patients. Cancer. 2001; 92:1759–1768. PMID:
11745247.

24. Reichardt P, Von Minckwitz G, Thuss-Patience PC, Jonat W, Kölbl H, Jänicke F, et al. Multicenter phase II study of oral capecitabine (Xeloda(")) in patients with metastatic breast cancer relapsing after treatment with a taxane-containing therapy. Ann Oncol. 2003; 14:1227–1233. PMID:
12881384.

25. Zambetti M, Bonadonna G, Valagussa P, Daidone MG, Coradini D, Bignami P, et al. Adjuvant CMF for node-negative and estrogen receptor-negative breast cancer patients. J Natl Cancer Inst Monogr. 1992; (11):77–83. PMID:
1627434.
26. Cleator S, Heller W, Coombes RC. Triple-negative breast cancer: therapeutic options. Lancet Oncol. 2007; 8:235–244. PMID:
17329194.

27. Rhee J, Han SW, Oh DY, Kim JH, Im SA, Han W, et al. The clinicopathologic characteristics and prognostic significance of triple-negativity in node-negative breast cancer. BMC Cancer. 2008; 8:307. PMID:
18947390.

28. Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007; 13(15 Pt 1):4429–4434. PMID:
17671126.

29. Paik S, Bryant J, Tan-Chiu E, Yothers G, Park C, Wickerham DL, et al. HER2 and choice of adjuvant chemotherapy for invasive breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-15. J Natl Cancer Inst. 2000; 92:1991–1998. PMID:
11121461.

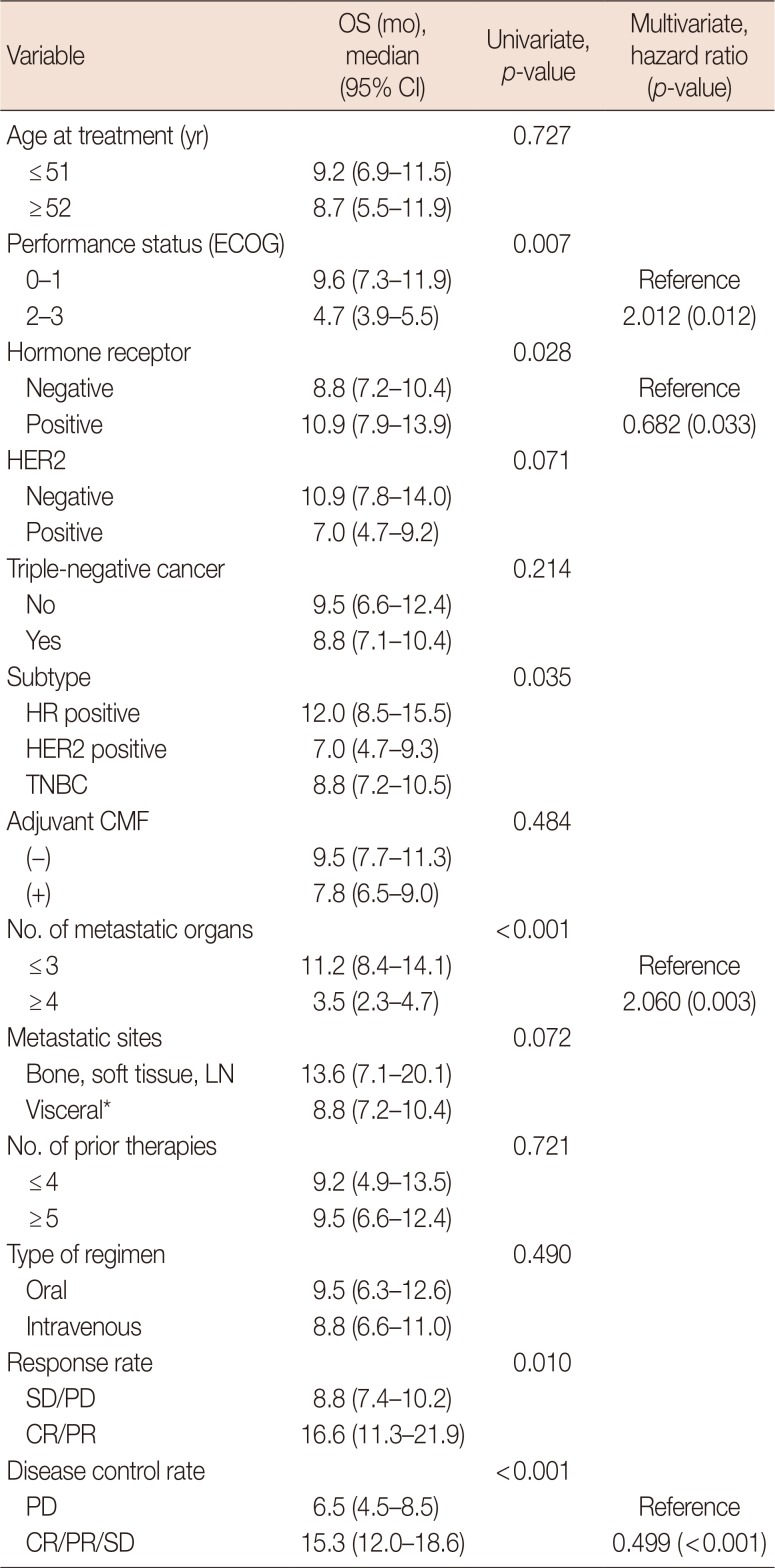




 PDF
PDF ePub
ePub Citation
Citation Print
Print


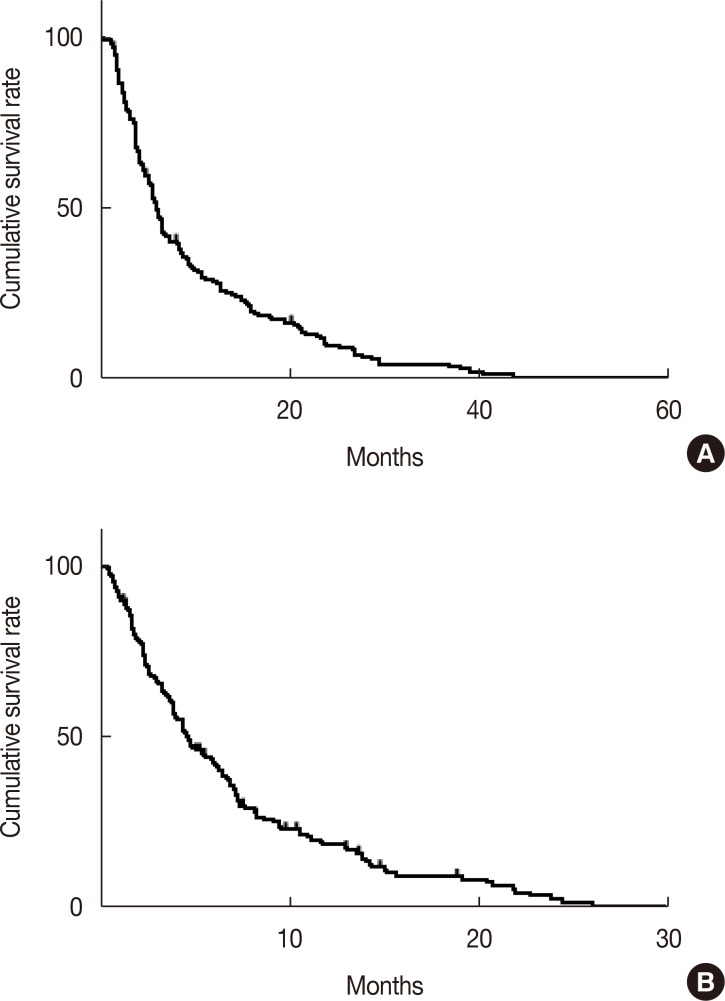
 XML Download
XML Download