Abstract
The development of ectopic breast tissue is attributable to the failure of primitive mammary tissue to regress after the development of the mammary ridge, except at pectoral breast sites, and is most often evident in the axillae. Several benign and malignant breast diseases have been reported in ectopic axillary breast tissues. The most common cancerous pathology of ectopic breast tissue is invasive ductal carcinoma. Ectopic breast cancer presenting with simultaneous primary cancer of the pectoral breast is extremely rare. Herein, we report an invasive micropapillary carcinoma of an axillary ectopic breast, combined with a synchronous ductal carcinoma in situ in the contralateral pectoral breast of a 61-year-old woman.
Breast development commences at week 5 or 6 of gestation. Ectopic breast tissue, also known as accessory breast tissue, arises when the primitive mammary tissue fails to regress after the development of the mammary ridge (except in the region of the pectoral breasts) [1]. Ectopic breast tissue is subject to the same physiological and pathological changes as normal pectoral breasts, and various benign and malignant lesions are thus observed in ectopic breast tissues [2].
Malignancies in ectopic breast tissues account for 0.2% to 0.6% of all breast cancers [34]. Ectopic breast cancers may occur synchronously with pectoral breast cancers [56]. However, cancers of the axillary ectopic breast concurrent with pectoral breast cancer are extremely rare, and only a few cases have been reported [7]. Moreover, invasive micropapillary carcinoma in ectopic breast tissue has not yet been reported. We herein describe an invasive micropapillary carcinoma developing in an axillary ectopic breast and synchronous ductal carcinoma in situ in the contralateral pectoral breast of a 61-year-old woman, along with the imaging and pathological findings and a literature review.
A 61-year-old woman visited a local breast clinic complaining of a palpable mass in her right axilla. The axillary mass was locally excised after ultrasound-guided fine-needle aspiration, which revealed cellular atypia. The pathological diagnosis was of a poorly differentiated adenocarcinoma. The patient was subsequently referred to Chonnam National University Hwasun Hospital.
Bilateral mammograms performed at a local breast clinic were retrieved and reviewed. Focal asymmetry and a mass with microcalcifications were evident in the right axilla. Axillary fibroglandular tissues, similar in radiological appearance to normal breast tissue (and thus compatible with ectopic breast tissue), were evident in both axillae (Figure 1). Incidentally, grouped calcifications were seen in the left breast. Subsequent magnified views were obtained and grouped fine pleomorphic microcalcifications were seen in the upper outer quadrant of the left breast (Figure 2). Breast ultrasonography was subsequently performed. Postexcisional changes, with residual small hypoechoic masses, were apparent in the right axilla and accessory breast tissue was evident in both axillae. No suspicious ultrasonographic findings were apparent in either breast. Ultrasonographic images obtained at a local breast clinic were reviewed; accessory breast tissues surrounding hypoechoic masses were evident (Figure 3). The right axillary masses were considered to be breast carcinomas arising in the ectopic breast tissue. Re-examination of paraffin-embedded tissue revealed focally abundant breast tissue, consisting primarily of breast lobules adjacent to invasive carcinoma. Wirelocalized excision biopsy under mammographic guidance was performed for the suspicious calcifications in the left breast; the biopsy revealed ductal carcinoma in situ. Breast magnetic resonance imaging was performed and there were no suspicious findings, with the exception of postexcisional changes in the right axilla and the left breast. Wide excision of the right axillary masses, the ectopic breast, and regional lymph nodes were performed. Conserving surgery of the left breast was also performed.
Pathological examination of the right axillary masses revealed invasive micropapillary carcinoma within ectopic breast tissue (Figure 4). Microscopically, the tumor was composed of morule-like clusters within spaces defined by networks of loose fibrocollagenous stroma. Micropapillary ductal carcinoma in situ was also present. Axillary tissues surrounding the tumor showed glandular structures reminiscent of mammary lobules. Immunohistochemical staining was carried out with a BenchMark XT autostainer (Ventana Medical Systems, Tucson, USA). The findings revealed that the carcinoma cells were negative for estrogen receptors (clone SP1, prediluted; Ventana Medical Systems) and progesterone receptors (clone 1E2, prediluted; Ventana Medical Systems), but positive for human epidermal growth factor receptor 2 (HER2) (clone 4B5, prediluted; Ventana Medical Systems). One metastatic lymph node was noted in the right axilla. The pathological findings allowed us to define the case as an invasive micropapillary carcinoma in an axillary ectopic breast. In the left breast, a high-grade ductal carcinoma in situ with microcalcifications and comedo-type necrosis was discovered (Figure 4). The lesion was 12 mm in size. The carcinoma cells were positive for estrogen and progesterone receptors, and HER2.
After surgery, four cycles of doxorubicin (60 mg/m2) and cyclophosphamide (600 mg/m2), followed by four cycles of docetaxel (75 mg/m2) were administered intravenously every 3 weeks. Accomplishing radiotherapy, she was scheduled to receive 1 year of trastuzumab therapy and 5 years of aromatase inhibitor hormone therapy. At the 12-month follow-up, no recurrence was evident.
Breast tissue develops from mammary ridges (or milk lines) that extend from the anterior axillary folds to the inside of the inguinal folds. Mammary ridge regression failure creates ectopic breast tissue with varying degrees of clinical expression including breast tissue with a nipple lacking an areola, breast tissue with an areola but lacking a nipple, or only breast tissue with neither an areola nor a nipple. Ectopic breast tissue may be found anywhere along the line; the axilla is the site most commonly involved [8]. Ectopic breast tissue may be subcutaneous. Knowledge about ectopic breast tissue has important implications for patient care. If a subcutaneous mass is evident along the mammary line, the possibility that the mass is attributable to ectopic breast tissue should be considered. In addition, in patients diagnosed with breast cancer, axillary ectopic breast tissue and/or mastopathy of the ectopic breast should be considered during differential diagnosis of lymph node metastasis; this may prevent unnecessary surgery [9].
Ultrasonography is the primary imaging modality used to evaluate ectopic breast tissue. Sonographically, the appearance of ectopic breast tissue is the same as that of tissue within the breast; fibroductal tissue and lobules of fat can be seen. Mammographically, axillary fibroglandular tissue interspersed with fat will have the same radiological appearance as normal breast tissue, but will be separate from the main breast parenchyma [10]. Ectopic breast tissue is embryologically identical to normal breast tissue, and indeed responds similarly to hormones, thus rendering the tissue susceptible to the same diseases. Therefore, axillary ectopic breast tissue should be included during regular screening surveillance. If a suspicious mass or calcification is seen mammographically or ultrasonographically within ectopic breast tissue, tissue sampling should be performed. It may be difficult to distinguish an ectopic breast carcinoma from a sweat gland adenocarcinoma of the axilla, or axillary lymph node metastasis. Visualization of aberrant normal breast tissue lying adjacent to an axillary cancer, identification of estrogen and progesterone receptors, and the absence of lymphoid tissue can be used to differentiate a primary carcinoma of ectopic breast tissue from other conditions [11].
Malignancies in ectopic breast tissue account for 0.2% to 0.6% of all breast cancer cases [34]; 91.5% of all ectopic breast cancers develop in the axilla [12]. Ductal cancer is the most common type of ectopic breast cancer and invasive ductal cancer constitutes 75% to 79% of all ectopic breast malignancies. Medullary carcinoma, mucinous carcinoma, apocrine carcinoma, lobular cancer, and Paget's disease also occur [412]. As in our case, invasive micropapillary carcinoma is a very rare histological form of breast cancer. Although this carcinoma is rare, it is clinically significant because of the high frequency of lymphovascular invasion, axillary nodal metastasis, and locoregional recurrence, as well as the poor prognosis [13]. It has been reported that mammographic, ultrasonographic, and magnetic resonance imaging findings of invasive micropapillary carcinoma are highly suggestive of malignancy and no feature to distinguish invasive micropapillary carcinoma from typical invasive ductal carcinoma has yet been described [14].
Concurrent axillary ectopic breast cancer and pectoral breast cancer is extremely rare; only one case report with ectopic breast cancer concomitant with bilateral, primary invasive breast carcinoma has been described to date [7]. In our present case, the contralateral pectoral breast cancer was incidentally detected. Furthermore, both the histological type and expression profile of axillary ectopic breast cancer and the pectoral breast carcinoma differed, suggesting that the primary origin of the carcinoma in the contralateral breast differed from that of the axilla. Although it is rare, preoperative evaluation of both pectoral breasts should be performed in patients with an ectopic breast cancer in the axilla.
The treatment recommendations for ectopic breast cancer are based on the guidelines used for tumor staging of pectoral breast cancer. The surgical procedure of choice that has been suggested for ectopic breast carcinoma is wide resection of the tumor and surrounding tissue, as well as the covering skin and regional lymph nodes [6]. Ipsilateral mastectomy, in addition to axillary lymph node dissection, was not superior to local excision with node dissection [3]. Adjuvant therapies (e.g., chemotherapy, hormonal therapy, and irradiation) are being used to reduce recurrence rates and improve the survival of patients with ectopic breast cancer [3]. It has been reported that the outcomes of ectopic breast cancer are poorer than those of pectoral breast cancer, because the tumors are located near the axillary lymph nodes and thus are associated with early metastasis to these nodes [15]. However, not all ectopic breast cancers arising in the axilla are associated with a higher risk of nodal metastases. It has been suggested that the poorer outcomes are due to a lack of awareness in the medical community and a delayed diagnosis at more advanced stages [12]. Further studies are needed to assess prognosis and long-term survival.
In summary, we report a case of invasive micropapillary carcinoma in an axillary ectopic breast and synchronous ductal carcinoma in situ in the contralateral pectoral breast. Although axillary ectopic breast cancer is relatively rare, it should be considered in the differential diagnosis of an axillary mass to avoid any delay in diagnosis and treatment. In addition, evaluation of both pectoral breasts is essential in patients with ectopic breast cancers.
Figures and Tables
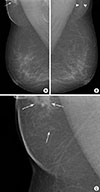 | Figure 1Mammographic findings of invasive micropapillary carcinoma in axillary ectopic breast. (A) Mediolateral oblique view of mammogram shows focal asymmetry with microcalcifications (arrow) in the right axilla. (B) Axillary fibroglandular tissue with the same radiological appearance as normal breast tissue (arrowheads) is also seen in the left axilla. (C) Zoomed mediolateral oblique view of right mammogram shows focal asymmetry and mass with microcalcifications (arrows) in the axilla. |
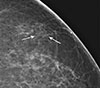 | Figure 2Mammographic findings of left breast. Magnified craniocaudal view of mammogram shows grouped, fine pleomorphic microcalcifications (arrows) in the upper outer quadrant of the left breast. |
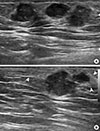 | Figure 3Sonographic findings of invasive micropapillary carcinoma in axillary ectopic breast. (A) Ultrasound image obtained in a local breast clinic shows several, irregular hypoechoic masses in the right axilla. (B) Echogenic area with the same appearance as that of normal breast tissue compatible with ectopic breast (arrowheads) is seen around the masses. |
 | Figure 4Histopathological findings. (A) Invasive micropapillary carcinoma and micropapillary ductal carcinoma in situ (arrows) are seen within the fatty mammary stroma with mammary lobules (arrowheads) in the right axilla (H&E stain, ×20). (B) Ductal carcinoma in situ with central necrosis and calcifications is seen in the left breast (H&E stain, ×200). |
References
1. De Cholnoky T. Accessory breast tissue in the axilla. N Y State J Med. 1951; 51:2245–2248.
2. Kim EY, Ko EY, Han BK, Shin JH, Hahn SY, Kang SS, et al. Sonography of axillary masses: what should be considered other than the lymph nodes? J Ultrasound Med. 2009; 28:923–939.
4. Caceres M, Shih J, Eckert M, Gardner R. Metaplastic carcinoma in an ectopic breast. South Med J. 2002; 95:462–466.

5. Guerry RL, Pratt-Thomas HR. Carcinoma of supernumerary breast of vulva with bilateral mammary cancer. Cancer. 1976; 38:2570–2574.

6. Marshall MB, Moynihan JJ, Frost A, Evans SR. Ectopic breast cancer: case report and literature review. Surg Oncol. 1994; 3:295–304.

7. Hao JY, Yang CC, Liu FF, Yang YL, Li S, Li WD, et al. Accessory breast cancer occurring concurrently with bilateral primary invasive breast carcinomas: a report of two cases and literature review. Cancer Biol Med. 2012; 9:197–201.
9. Kitamura K, Kuwano H, Kiyomatsu K, Ikejiri K, Sugimachi K, Saku M. Mastopathy of the accessory breast in the bilateral axillary regions occurring concurrently with advanced breast cancer. Breast Cancer Res Treat. 1995; 35:221–224.

10. DeFilippis EM, Arleo EK. The ABCs of accessory breast tissue: basic information every radiologist should know. AJR Am J Roentgenol. 2014; 202:1157–1162.

11. Yerra L, Karnad AB, Votaw ML. Primary breast cancer in aberrant breast tissue in the axilla. South Med J. 1997; 90:661–662.

12. Nihon-Yanagi Y, Ueda T, Kameda N, Okazumi S. A case of ectopic breast cancer with a literature review. Surg Oncol. 2011; 20:35–42.

13. Kuroda H, Sakamoto G, Ohnisi K, Itoyama S. Clinical and pathologic features of invasive micropapillary carcinoma. Breast Cancer. 2004; 11:169–174.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download