Abstract
We report a case of chronic myeloid leukemia (CML) that developed after postoperative chemotherapy with cyclophosphamide, doxorubicin and 5-fluorouracil (CAF) for breast cancer. A 55-year-old woman was diagnosed with invasive ductal carcinoma which was treated with a modified radical mastectomy followed by six cycles of CAF chemotherapy. Nine years later, she developed CML and locoregional recurrence. Her breast recurrence showed strong estrogen receptor, weak progesterone receptor and strong human epidermal growth factor 2 (score 3+) expression. Her secondary CML in the chronic phase showed a complex variant translocation (CVT) involving chromosomes 9, 22, and 17. Considering that the HER2/neu gene is also located on chromosome 17, this secondary CML in chronic phase with CVT is indeed a rare occurrence. We discuss the associated genetic factors and the possible role of breast cancer chemo/radiotherapy in the development of such CML as well as its treatment and prognosis compared with de novo CML.
Chronic myeloid leukemia (CML) accounts for a small percentage of secondary leukemias. Secondary leukemias that develop after invasive ductal carcinoma treatment are mostly acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). There is a 1% to 5% lifetime risk of developing therapy-related myeloid neoplasms (t-MN) after breast cancer treatment [1]. We report a rare case in which CML with a complex variant translocation (CVT) developed after postoperative chemotherapy for breast cancer.
A 55-year-old postmenopausal woman presented at our oncology tertiary referral center with a right sternal hard swelling that had increased in size over the past 2 months and measured 2×2 cm. She underwent a right modified radical mastectomy 9 years previously for invasive ductal carcinoma and received six cycles of chemotherapy with cyclophosphamide, doxorubicin and 5-fluorouracil (CAF). Fine needle aspiration cytology of a chest wall nodule revealed features of ductal carcinoma. The patient's complete blood count at presentation was 9.3 g/dL hemoglobin, the white blood cell (WBC) count was 92.14×103/µL and the platelet count was 675×103/µL. A peripheral blood smear had a differential leucocyte count of 9% myelocytes, 17% metamyelocytes, 72% neutrophils, 1% lymphocytes, and 1% eosinophils. The possibility of a chronic myeloproliferative neoplasm was considered. Contrast-enhanced computed tomography revealed a metastatic lesion involving the sternum with right internal mammary, pretracheal and paratracheal lymphadenopathy.
Analysis of a trucut biopsy from the chest wall resulted in a diagnosis of infiltrating ductal carcinoma- not otherwise specified, grade 2 (Figure 1A). Hormone receptor studies revealed strong nuclear estrogen receptor positivity in >90% of tumor cells and weak nuclear progesterone receptor positivity in 1% of tumor cells. Human epidermal growth factor 2 (HER2) was strongly positive with a-score of 3+ (Figure 1B).
Bone marrow aspiration and biopsy indicated chronic myeloproliferative neoplasm, and more specifically chronic myeloid leukemia in chronic phase (CML-CP) (Figure 1C).
Chromosomal analysis using conventional cytogenetics showed complex reciprocal translocation between the long arms of chromosomes 9 and 22 and the short arm of chromosome 17, between regions q34, q11.2, and p12. This was suggestive of “Ph” positive chromosome (variant) complement (karyotype: 46,XX,t[9;22;17][q34;q11.2;p12]) (Figure 1D). The patient's white blood cell count gradually increased from 92.14×103/µL at presentation to 237.78×103/µL- 103 has to be superscripted not subscripted.
The patient received docetaxel, carboplatin and ifosfamide-based chemotherapy and hormonal therapy in the form of tamoxifen for the chest wall recurrence of ductal carcinoma and imatinib in tablet form for CML. The chest wall nodule responded to three cycles of chemotherapy, as confirmed by contrast-enhanced computed tomography. The patient received 45 Gy in 10 fractions by external beam to the right chest wall followed by 15 Gy in five fractions as a boost to the chest wall nodule. Her CML responded to imatinib as she achieved a hematological response in 3 weeks of treatment and had undetectable minimal residual disease based on real-time polymerase chain reaction (PCR) analysis within 1 year.
Breast cancer treatment has been associated with a 1% to 5% lifetime risk of t-MN [1]. The most common types of t-MN in the World Health Organization classification include AML, MDS, and myelodysplastic/myeloproliferative neoplasms, with the postcytotoxic ther apy incidence of CML being markedly lower [2].
CML is described as a carcinogenic effect of ionizing radiation, especially in atomic bomb survivors [3]. CML that develops after breast cancer treatment is rare. Two patients with CML in a registry cohort analysis of 5,790 breast cancer patients presented with stage I disease and were treated with surgery and radiation without chemotherapy, thus implicating the role of radiotherapy [4]. The risk associated with radiation was significant even for radiotherapy limited to the breast, as seen in a case control study including 182 AML and MDS patients and 534 matched controls [5]. Radiation can be excluded as a risk factor in our case as the patient only received CAF-based chemotherapy postsurgery.
Secondary leukemias that develop after breast cancer treatment are mostly AML and MDS. Amongst chemotherapeutic agents, topoisomerase-II inhibitors (mitoxantrone, anthracyclines, and epipodophyllotoxins) are significantly associated with an increased risk of developing these leukemias, as compared with alkylating agents such as cyclophosphamide. The association between anthracyclines and AML/MDS is of major importance because anthracycline based chemotherapy is currently the gold standard for breast cancer treatment [5]. In contrast radiation and chemotherapy might have a synergistic effect in the development of secondary AML/MDS, as proposed in a previous study [4].
Therapy-related CML cases are increasing, however, only a handful of cases of CML in patients treated for breast cancer have been reported [4678]. In a study of 15 cases of secondary CML at the Memorial Sloan-Kettering Cancer Center, New York, 12 patients received adjuvant radiation, 11 received adjuvant chemotherapy, and 15 received both therapies to the breast. The cumulative dose of anthracycline was 240 mg/m2 while that for alkylator was 4,800 mg/m2 [8]. In another such case report, two patients received cyclophosphamide, epirubicin/doxorubicin (anthracycline) as chemotherapy with axillary radiation applied in one case [7]. Our patient received CAF-based chemotherapy without radiation, thus implicating the role of chemotherapy in the development of secondary CML.
A classical Ph translocation t(9;22)(q34;q11.2) is observed in the majority of CML patients, with only 5% to 10% having variant Ph translocations. These variants are classified as simple variants involving chromosome 22 and a chromosome other than 9, or as complex variants that involve chromosomes 9, 22, and 1 or more other chromosomes [9]. Molecular methods or fluorescence in situ hybridization can be used to detect breakpoint cluster region-Abelson murine leukemia viral oncogene homolog 1 (BCR-ABL1) rearrangement in almost all cases with the variant Ph chromosome. In our case, the patient had CVT involving chromosomes 9, 22, and 17 which was detected using G-banded karyotyping.
Recurrent invasive ductal carcinoma in this case was found to be strongly HER2 positive using immunohistochemistry, which is associated with HER2/neu gene amplification on chromosome 17 [10]. The peculiarity of this case was the simultaneous relapse of invasive ductal carcinoma as bony metastasis and the development of secondary CML with CVT involving chromosome 17, which also harbors the HER2/neu gene. To the best of our knowledge, this is the first such reported case in the literature. Two such cases have been reported where breast cancer recurred as vertebral metastasis: however the concurrent CML had a typical “Ph” chromosome on cytogenetic analysis [7].
Imatinib was used as a frontline therapy in the treatment of CML-CP that developed after breast cancer treatment [78]. A large series of 559 early CML-CP patients treated with imatinib as a frontline therapy, which included 30 patients with variant translocations, concluded that the clinical characteristics and outcome of patients with variant Ph translocations are similar to those of patients with classic Ph translocations. The same study showed that patients with variant translocations do not constitute a “warning” category in the imatinib era [11]. We also observed this finding as our patient achieved a hematological response in 3 weeks of treatment and had undetectable minimal residual disease according to real-time-PCR within 1 year.
To conclude, we therefore infer that breast cancer chemotherapy (CAF) may have an independent role in the development of CML, independently of the well-recognized role of radiation. Hence, complete blood counts should be assessed in the follow-up of all breast cancer patients after treatment. The present case showed that imatinib therapy was as effective for secondary CML with CVT as it is for de novo CML, and that clinical and biological characteristic did not significantly differ between the two diseases. The exact pathogenesis of CML after adjuvant treatment for breast cancer and the reason why patients more commonly develop t-AML require further investigation.
Figures and Tables
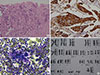 | Figure 1Microscopic findings and karyotyping. (A) Trucut biopsy sternal mass: recurrent duct carcinoma, breast (H&E stain, ×200). (B) Immunohistochemistry (IHC) for human epidermal growth factor receptor 2 (HER2), sternal mass shows 3+ positivity (IHC for HER2, ×400). (C) Bone marrow aspirate: chronic myeloid leukemia chronic phase, showing myeloid preponderance (May-Grünwald-Giemsa stain, ×400). (D) G-banded karyotyping: chronic myeloid leukemia with complex variant translocation involving chromosomes 9, 22, and 17. |
ACKNOWLEDGMENTS
We acknowledge Dr. M. Sujatha, Cytogeneticist, Vimta Labs Ltd, Cherlapali, Hyderabad for performing chromosomal analysis in this case.
References
1. Leone G, Fianchi L, Pagano L, Voso MT. Incidence and susceptibility to therapy-related myeloid neoplasms. Chem Biol Interact. 2010; 184:39–45.

2. Vardiman JW, Arber DA, Brunning RD, Larson RA, Matutes E, Baumann I, et al. Therapy-related myeloid neoplasms. In : Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon: IARC Press;2008. p. 127–129.
3. Preston DL, Kusumi S, Tomonaga M, Izumi S, Ron E, Kuramoto A, et al. Cancer incidence in atomic bomb survivors. Part III. Leukemia, lymphoma and multiple myeloma, 1950-1987. Radiat Res. 1994; 137:2 Suppl. S68–S97.

4. Kaplan HG, Malmgren JA, Atwood MK. Increased incidence of myelodysplastic syndrome and acute myeloid leukemia following breast cancer treatment with radiation alone or combined with chemotherapy: a registry cohort analysis 1990-2005. BMC Cancer. 2011; 11:260.

5. Le Deley MC, Suzan F, Cutuli B, Delaloge S, Shamsaldin A, Linassier C, et al. Anthracyclines, mitoxantrone, radiotherapy, and granulocyte colony-stimulating factor: risk factors for leukemia and myelodysplastic syndrome after breast cancer. J Clin Oncol. 2007; 25:292–300.

6. Setoodeh R, Zhang L. Chronic myelogenous leukemia following breast carcinoma: report of two cases and review of the literature. Am J Clin Pathol. 2012; 138:Suppl 1. A170.

7. Ocakçı S, Görümlü G, Şahin F, Özsan N, Zekioğlu O, Uslu R, et al. Occurrence of chronic myeloid leukemia in two breast cancer survivors after 4 years. Ege J Med. 2011; 50:141–144.
8. Bowman IA, Tallman MS, Douer D, Berman E, Maslak PG, Brentjens RJ, et al. Chronic myeloid leukemia after adjuvant treatment for breast cancer: is it therapy related? Blood. 2013; 122:2740.

9. Huret JL. Complex translocations, simple variant translocations and Ph-negative cases in chronic myelogenous leukaemia. Hum Genet. 1990; 85:565–568.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download