Abstract
Purpose
Intraoperative frozen-section analysis of the lumpect-omy margin during breast-conserving surgery (BCS) is an excellent method in obtaining a clear resection margin. This study aimed to investigate the usefulness of intraoperative circumferential frozen-section analysis (IOCFS) of lumpectomy margin during BCS for breast cancer, and to find factors that increase the conversion into mastectomy.
Methods
From 2007 to 2011, 509 patients with breast cancer underwent IOCFS during BCS. The outer surfaces of the shaved lumpectomy margins were evaluated. A negative margin was defined as no ink on the tumor. All margins were evaluated using the permanent section analysis.
Results
Among the 509 patients, 437 (85.9%) underwent BCS and 72 (14.1%) finally underwent mastectomy. Of the 483 pathologically confirmed patients, 338 (70.0%) were true-negative, 24 (5.0%) false-negative, 120 (24.8%) true-positive, and 1 (0.2%) false-positive. Twenty-four patients (4.7%) among total 509 patients had undetermined margins as either atypical ductal hyperplasia or ductal carcinoma in situ in the first IOCFS. The IOCFS has an accuracy of 94.8% with 83% sensitivity, 99.7% specificity, 93.4% negative predictive value, and 99.2% positive predictive value. Sixty-three cases (12.4%) were converted to mastectomy, the first intraoperatively. Of the 446 (87.6%) patients who successfully underwent BCS, 64 patients received additional excisions and 32 were reoperated to achieve clear margin (reoperation rate, 6.3%). Twenty-three of the reoperated patients underwent re-excisions using the second intraoperative frozen section analysis, and achieved BCS. Nine cases were additionally converted to mastectomy. No significant differences in age, stage, and biological factors were found between the BCS and mastectomy cases. Factors such as invasive lobular carcinoma, multiple tumors, large tumor, and multiple excisions increased the conversion to mastectomy.
Breast-conserving surgery (BCS), along with mastectomy, has been a standard treatment for breast cancers. BCS followed by radiotherapy provides better cosmetic outcomes and similar long-term survival rate as mastectomy [12]. The biggest drawback of BCS is the risk for local recurrence of the preserved breast parenchyma. The most important factor to prevent local recurrence, aside from adjuvant radiotherapy and systemic therapy, is the microscopic clearance of the resection margin during lumpectomy [3456]. Surgeons have performed intraoperative frozen-section analysis while performing BCS to obtain a clear resection margin and prevent reoperation. Reoperation confers psychological, physical, and financial burdens on patients, and potentially delays subsequent treatment. Reoperation rates vary among the reports, ranging from 10% to 50% [789].
Other intraoperative assessment methods of margins include touch preparation cytology, intraoperative ultrasonography, intraoperative mammography, and micro-computed tomography [10]. Among these methods, intraoperative frozen-section analysis (IOFSA) is the most reliable and effective to prevent reoperation [1112131415].
The IOFSA method is performed as follows: analyzing the resection margin of the lumpectomy specimen; analyzing the margin of the cavity; analyzing a portion of the resection margin after separately excising and marking the direction of the analysis; or analyzing the resection margin after performing a shaved excision of the resection margin. However, the technical standard for IOFSA has not yet been established, and the rates of tumor-positive margin and reoperation varied between various reports that used different methods [789].
This study aimed to investigate the accuracy of intraoperative circumferential frozen-section analysis (IOCFS) of lumpectomy margin during BCS and to determine its effects on reoperation. Moreover, we aimed to identify the factors that increase the conversion of BCS into mastectomy.
We performed an IOCFS on the lumpectomy margin in all 509 patients with breast cancer who underwent a BCS between 2007 and 2009 at the Cheil General Hospital & Women's Healthcare Center. BCS candidates were identified based on the findings from clinical and radiographic evaluations performed prior to surgery. The informed consents were also obtained prior to surgery, and the surgeons decided whether further excision or conversion to mastectomy should be done based on the IOCFS result. This study was approved by the Institutional Review Board of the Cheil General Hospital & Women's Healthcare Center (CGH-IRB-2017-25).
We aimed for a grossly negative 1 cm margin during the first lumpectomy. For nonpalpable lesions, mammography- or ultrasonography-guided needle localization was performed, preoperatively. When microcalcifications in the tumor were detected on preoperative mammography, we performed the specimen mammography to ensure a precise excision. If the calcifications were close to the margin in the specimen mammography, a wider excision was performed irrespective of the frozen-section result.
Sentinel lymph node biopsy was performed prior to BCS. In the first lumpectomy specimen, the directions were marked using a silk suture. Then, the specimen was sent to the pathology department and evaluated using the following method (Figure 1). The margin of the first lumpectomy specimen was shaved into 6 to 13 pieces circumferentially, which were shown alphabetically. The shaved margin was usually 5 mm thick. The outer surface of the shaved lumpectomy margin was marked with ink and assessed as frozen sections. A negative margin was defined as “no ink on the tumor” including carcinoma in situ and invasive carcinoma. Several sections with approximately 10 µm in thickness were stained with hematoxylin and eosin and microscopically evaluated. If a tumor was found in the outer surface of the margin, re-excision was performed, maintaining the direction and the 5-mm thickness from the edge of the cavity, and the outer surface was re-examined.
If tumor was involved in the resection margin under the IOCFS, the surgeon decided whether to perform further excision, to confirm permanent section results later, or to convert to mastectomy. If the shape of the breast had cosmetic problems or if malignant invasion was present in the nipples, the surgeon could convert to mastectomy even if no tumor was detected in the breast resection margin. All specimens obtained after the operation were formalin-fixed and paraffin embedded (permanent section), and the resection margin was re-examined.
All data were analyzed using IBM SPSS statistics version 19.0 software (IBM Corp., Armonk, USA). The chi-square test was used to compare the clinical features of all patients after dividing the patients into BCS group and mastectomy group. The t-test was used to compare the mean values of the continuous variables. The multivariate logistic regression model was used to analyze factors associated with conversion to mastectomy. For all analyses, a p-value of <0.05 was considered statistically significant.
To assess the accuracy of IOCFS on the lumpectomy margin, results were categorized as true-negative (TN), true-positive (TP), false-negative (FN), or false-positive (FP) margins after being compared with the permanent section results. The FN margin was defined as the absence of tumor in the frozen-section margin but was present in the permanent section margin.
Partial mastectomy was attempted in 509 patients, and IOCFS was performed in all the patients. Of the 509 patients, 437 (85.9%) underwent BCS and the remaining 72 (14.1%) ultimately underwent mastectomy. The median age of patients was 50 years (range, 28–77 years).
No significant difference in the mean age or the number of tissue blocks in the frozen section of lumpectomy margin was found in both BCS and mastectomy groups. The histologic type, tumor stage, histologic grade, estrogen receptor, progesterone receptor, or human epidermal growth factor receptor 2 (HER2) expression showed no significant difference between the two groups (Table 1).
However, the prevalence rates of ductal carcinoma in situ (DCIS) (11.9% vs. 15.3% for BCS and mastectomy) and invasive lobular carcinoma (1.6% vs. 9.7% for BCS and mastectomy) were higher in the mastectomy group. Tumor sizes ≥T2 were more prevalent in the mastectomy group. Specifically, the frequencies of 2 and ≥3 tumors were 9.7% (BCS group, 5.5%) and 18.1% (BCS group, 3.9%), respectively, demonstrating markedly higher frequencies in the mastectomy group. Further excisions resulted in an increased conversion to mastectomy (Table 1).
Turnaround time data were available for 53 patients operated recently. The median turnaround time per case was 40 minutes (range, 10–85 minutes). The median number of total tissue blocks submitted per case was 13 (range, 2–27).
Of the 509 patients, 24 (4.7%) had undetermined margins as either atypical ductal hyperplasia (ADH) or DCIS in the first IOCFS (described as undetermined margin-group). These were reported in 12 ADH, eight DCIS, one normal, and three cases that had disappeared in the permanent section. The undetermined margin-group patients were excluded in measuring the accuracy of IOCFS in lumpectomy margins.
One hundred twenty-three patients had positive margins after the initial lumpectomy with IOCFS. In two cases, the tumor cells in the permanent section had disappeared; therefore, they were excluded in measuring the accuracy of IOCFS. One of the 121 patients had a negative margin in the final pathology (FP case, 0.2% of 483), who had DCIS positive margin in the first IOCFS and ductal hyperplasia-confirmed margin in the permanent section. A total of 120 patients were confirmed to have tumor-positive margins in the final pathology (TP case, 24.8%) (Table 2).
Of the 362 patients with tumor-negative margin in IOFSA, 24 had positive margins in the final pathological examination (FN case, 5.0%). A total of 338 patients had negative margins in the permanent section (TN case, 70.0%) (Table 2).
Between the TN and FN groups, the prevalence of DCIS (TN, 10.4% vs. FN, 16.7%) and invasive lobular carcinoma (TN, 1.5% vs. FN, 8.3%) were higher in the FN group. Moreover, the frequencies of ≥3 tumors were 16.7% (TN group, 2.7%), demonstrating definitely higher frequencies in the FN group. The mean age, TNM stage, tumor size, histologic grade, or biologic markers (including estrogen receptor, progesterone receptor, HER2 expression) showed no significant difference between the two groups (Table 3). The description about histologic grade and biologic markers was not included in Table 3.
Sixty-three patients (12.4%) were intraoperatively converted to mastectomy based on the results of the additional resections. Of the 446 patients who successfully underwent BCS intraoperatively, 64 (14.3%) received additional excisions. A total of 414 (92.8%) patients had a margin clearance during the postoperative permanent section. Among the 446 patients, 32 (7.2%) were reoperated to achieve the clear resection margin (total reoperation rate, 6.3%), which was comprised of 24 FN and eight DCIS-confirmed patients of the undetermined margin-group; 23 (5.2%) were with FN results and DCIS-confirmed undetermined margin-group receiving additional re-excisions using the second IOFSA and achieving BCS intraoperatively; and nine (2%) were converted to mastectomy intraoperatively following the second IOFSA re-excision. Finally, a total of 72 cases (14.1%) were converted to mastectomy intraoperatively (Figure 2).
All patients were evaluated for the correlation between the IOCFS and final permanent section pathology (Table 2). The undetermined margin-group in the first IOCFS and two cases with absence of tumor cells in the permanent section were excluded in the accuracy measurement. The accuracy of the IOCFS of lumpectomy margins during BCS was 94.8% with 83.3% sensitivity, 99.7% specificity, and 93.4% negative and 99.2% positive predictive values, respectively.
The multivariate logistic regression revealed that the conversion to mastectomy was significantly associated with invasive lobular carcinoma (p=0.05), multifocality of ≥3 tumors (p=0.005), and further excisions (p=0.009). In addition, small tumor size (T1) significantly decreased the conversion into mastectomy (p=0.005) (Table 4).
In Korea, the proportion of patients undergoing BCS has increased over time, surpassing that of the mastectomy in 2006, and reaching 64.9% of the proportion in 2014 [16]. As the margin clearance of BCS is considered the most important key to local control, Korean surgeons usually use the intraoperative frozen-section analysis of lumpectomy margins as the preferred method in the clearance of resection margins.
In this study, we used intraoperative entire-circumferential frozen-section analysis to diagnose the lumpectomy margin status. The FN margin of our IOCFS was 5.0%, which was considerably low compared to the FN margins of IOFSA in other reports. The FN rate in other study was up to 18.6% [17]. The accuracy of IOFSA was reported to be around 83% to 98%, although it differs depending on the report [17181920]. In our study, the accuracy of IOCFS was as high as 94.8%, which seems to be due to the continuous evaluation of circumferential frozen section of lumpectomy margins (Table 5).
The reoperation rate of our study was 6.3%, which was relatively low (Table 5). We performed intraoperative re-excision to achieve final negative margins. The reoperated patients were composed of the FN cases and DCIS-confirmed patients of the undetermined margin-group. Depending on the investigator, second operation rates are reported to be as high as 40% using the assessment of lumpectomy margin [21]. In the United States, the IOFSA of lumpectomy margins is not widely used for margin analysis. However, there has been considerable evidence showing the feasibility, reliability, and safety of the IOFSA in margin assessment [11171822]. We could be sure of markedly reducing reoperations under the IOCFS of lumpectomy margins.
One of the disadvantages of IOFSA is its time-consuming. In our case, the first lumpectomy margin analysis took a median turnaround time of 40 minutes. Other investigators have reported need of approximately 50 minutes turnaround time in IOFSA cases on the entire shaved margins [1523], and 13 and 25 minutes for partial and separate excision analysis of margins, respectively [1718]. Although additional time may be required during operation, the IOFSA is nonetheless an accurate procedure to achieve clear resection margins and to prevent reoperation. Therefore, creating a standard method of IOFSA to reduce the turnaround time is important.
It has been reported that as IOFSA of lumpectomy margin collected fewer margins, the margin positivity became lower [23]. Huston et al. [24] reported an analysis that obtaining four to six cavity margins was diagnostically remarkable than obtaining one to three cavity margins. The correlation between the number of margin blocks and positivity was not analyzed in this study. If we analyzed the circumferential shaved margin, many margin pieces should be collected accordingly. The IOCSF of lumpectomy margins have a high diagnostic accuracy and low reoperation rate. However, since many margin pieces may be collected in such procedure, the margin positivity will increase. This may lead to further excisions and more cosmetic problems during BCS. Therefore, further investigations on how IOFSA should be performed and how many margin sections should be collected are necessary, in order to determine the most clinically and cosmetically sound procedure.
The conversion rate to mastectomy varies between institutions depending on the IOFSA method of lumpectomy margins. An author reported a considerably low conversion rate of 1.4% [15]. We demonstrated a conversion rate of 14.1%, which was comparable to, or lower than other reports [2325]. The subgroups that need mastectomy might exist in breast cancer patients. If we excessively proceed with BCS, it may lead to the cosmetic problems or increased ipsilateral breast tumor recurrence. Based on the multivariate logistic regression analysis in this study, the rate of conversion to mastect-omy increased in invasive lobular carcinoma, further excisions, and ≥3 multifocal tumors cases, whereas it decreased in small-sized tumor (T1) cases. These factors were similar to the specific features of FN cases, that is, invasive lobular carcinoma, DCIS, and multifocal tumors. If we preoperatively consider all these factors, we can predict a mastectomy case. Patients with preoperatively diagnosed invasive lobular carcinoma, DCIS, and multifocal tumors would be the subgroup that needs the IOFSA. Moreover, we could provide more information to patients and prepare the immediate reconstruction surgery.
The IOFSA of lumpectomy margin is the most useful method in assessing the clearance of resection margins. However, we believe that performing the IOFSA of lumpectomy margin in all patients undergoing BCS would not be necessary. Considering the positive rate of resection margins in the IOFSA, we can determine the subgroup who needs the IOFSA during BCS, which will beneficial to patients, surgeons, and pathologists in finding the subgroup and then in specifically performing the IOFSA.
We have some limitations in this study. We could not analyze the local recurrence or metastasis of ipsilateral breast cancers using this method. If the follow-up of this method is investigated, we can conclude that IOFSA is the best procedure in lumpectomy margins during BCS.
In conclusion, the IOCFS analysis of lumpectomy margins during BCS is useful in evaluating lumpectomy margins and preventing reoperation. Factors, such as invasive lobular carcinoma, multiple tumors, and multiple excisions, increased the conversion to mastectomy during the attempted BCS. Conversely, the small-sized tumors decreased the conversion to mastectomy. These factors need to be preoperatively considered to determine appropriate operations.
Figures and Tables
 | Figure 1Methods of intraoperative circumferential frozen-section analysis of lumpectomy margins during breast-conserving surgery. (A) The margin of the first lumpectomy specimen was shaved into 6–13 pieces circumferentially, which are shown in alphabetical order. (B) Each piece of shaved margins was usually 5 mm thick. The outer surfaces of the shaved lumpectomy margin were marked with ink and examined (red line in B). Several sections, approximately 10 µm thick, were stained with hematoxylin and eosin, and microscopically evaluated. (C) When the tumor (either invasive carcinoma or carcinoma in situ) extended to the inked margin of the c position (black line in C), the surgeons additionally resected the margin of the cavity with about 1 cm thick slices from the b position to the d position. The outer surface of the shaved cavitary margin was marked with ink and examined (red line in C). All margins were evaluated with postoperative permanent section. |
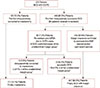 | Figure 2Final operations of attempted breast-conserving surgery (BCS) cases based on the intraoperative circumferential frozen-section analysis (IOCFS) of lumpectomy margins.FN=false negative; DCIS=ductal carcinoma in situ; ADH=atypical ductal hyperplasia; IOFSA=intraoperative frozen-section analysis. *the undetermined margin-group, patients diagnosed as undetermined margins in the first IOCFS.
|
Table 1
Characteristics of patients who underwent breast-conserving surgery with intraoperative circumferential frozen section analysis

BCS=breast-conserving surgery; DCIS=ductal carcinoma in situ; IDC= invasive ductal carcinoma; ILC=invasive lobular carcinoma; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2; FSM=frozen section of margin.
*Mean±SD; †Missing value of tumor stage: 2 cases; ‡Histologic grade: unknown cases were excluded as missing value (BCS cases, 56; mastectomy cases, 12).
Table 2
The cross table to assess the accuracy of intraoperative circumferential frozen section analysis of lumpectomy margin

| FSM | Permanent pathology of margin, No. (%) | Total | |
|---|---|---|---|
| Tumor-positive | Tumor-negative | ||
| Tumor-positive | 120 (24.8)* | 1 (0.2)† | 121 |
| Tumor-negative | 24 (5.0)‡ | 338 (70.0)§ | 362 |
| Total | 144 | 339 | 483 |
Table 3
The characteristics of patients with true negative margins and false negative margins in the intraoperative circumferential frozen section analysis of lumpectomy

Table 4
Multivariate logistic regression analysis for the clinical characteristics associated with the conversion into mastectomy during breast-conserving surgery

Table 5
Accuracy and reoperation rate for various intraoperative margin assessment techniques
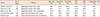
| Author | No. of cases | Method | Accuracy (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Reoperation rate (%) |
|---|---|---|---|---|---|---|---|---|
| Cendán et al. [17] | 97 | Separately excision from cavity | 96.0 | 64.8 | 100.0 | 94.0 | 78.0 | 19.6 |
| Olson et al. [18] | 290 | Separately excision from cavity | 98.0 | 73.1 | 99.6 | 91.9 | 98.3 | 5.5 |
| Weber et al. [19] | 115 | Separately excision from lumpectomy | 83.8 | 80.0 | 87.5 | 86.5 | 81.4 | 12.5 |
| Fukamachi et al. [20] | 122 | Circumferential excision from cavity | 95.1 | 78.6 | 100.0 | 100.0 | 94.0 | 9.8 |
| Present study | 509 | Circumferential excision from lumpectomy | 94.8 | 83.3 | 99.7 | 99.2 | 93.4 | 6.3 |
ACKNOWLEDGMENTS
We would like to thank doctors Sung Ran Hong, Hye Sun Kim, and Hae Kyung Lee at the Cheil General Hospital & Women's Healthcare Center.
References
1. van Dongen JA, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, Tong D, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000; 92:1143–1150.

2. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002; 347:1233–1241.

3. Gage I, Schnitt SJ, Nixon AJ, Silver B, Recht A, Troyan SL, et al. Pathologic margin involvement and the risk of recurrence in patients treated with breast-conserving therapy. Cancer. 1996; 78:1921–1928.

4. Leong C, Boyages J, Jayasinghe UW, Bilous M, Ung O, Chua B, et al. Effect of margins on ipsilateral breast tumor recurrence after breast conservation therapy for lymph node-negative breast carcinoma. Cancer. 2004; 100:1823–1832.

5. Schnitt SJ, Abner A, Gelman R, Connolly JL, Recht A, Duda RB, et al. The relationship between microscopic margins of resection and the risk of local recurrence in patients with breast cancer treated with breast-conserving surgery and radiation therapy. Cancer. 1994; 74:1746–1751.

6. Smitt MC, Nowels KW, Zdeblick MJ, Jeffrey S, Carlson RW, Stockdale FE, et al. The importance of the lumpectomy surgical margin status in long-term results of breast conservation. Cancer. 1995; 76:259–267.

7. Aziz D, Rawlinson E, Narod SA, Sun P, Lickley HL, McCready DR, et al. The role of reexcision for positive margins in optimizing local disease control after breast-conserving surgery for cancer. Breast J. 2006; 12:331–337.

8. Talsma AK, Reedijk AM, Damhuis RA, Westenend PJ, Vles WJ. Re-resection rates after breast-conserving surgery as a performance indicator: introduction of a case-mix model to allow comparison between Dutch hospitals. Eur J Surg Oncol. 2011; 37:357–363.

9. Jacobson AF, Asad J, Boolbol SK, Osborne MP, Boachie-Adjei K, Feldman SM. Do additional shaved margins at the time of lumpectomy eliminate the need for re-excision? Am J Surg. 2008; 196:556–558.

10. Thill M, Baumann K, Barinoff J. Intraoperative assessment of margins in breast conservative surgery: still in use? J Surg Oncol. 2014; 110:15–20.

11. Cabioglu N, Hunt KK, Sahin AA, Kuerer HM, Babiera GV, Singletary SE, et al. Role for intraoperative margin assessment in patients undergoing breast-conserving surgery. Ann Surg Oncol. 2007; 14:1458–1471.

12. Camp ER, McAuliffe PF, Gilroy JS, Morris CG, Lind DS, Mendenhall NP, et al. Minimizing local recurrence after breast conserving therapy using intraoperative shaved margins to determine pathologic tumor clearance. J Am Coll Surg. 2005; 201:855–861.

13. Cao D, Lin C, Woo SH, Vang R, Tsangaris TN, Argani P. Separate cavity margin sampling at the time of initial breast lumpectomy significantly reduces the need for reexcisions. Am J Surg Pathol. 2005; 29:1625–1632.

14. Jorns JM, Visscher D, Sabel M, Breslin T, Healy P, Daignaut S, et al. Intraoperative frozen section analysis of margins in breast conserving surgery significantly decreases reoperative rates: one-year experience at an ambulatory surgical center. Am J Clin Pathol. 2012; 138:657–669.

15. Osako T, Nishimura R, Nishiyama Y, Okumura Y, Tashima R, Nakano M, et al. Efficacy of intraoperative entire-circumferential frozen section analysis of lumpectomy margins during breast-conserving surgery for breast cancer. Int J Clin Oncol. 2015; 20:1093–1101.

16. Park EH, Min SY, Kim Z, Yoon CS, Jung KW, Nam SJ, et al. Basic facts of breast cancer in Korea in 2014: the 10-year overall survival progress. J Breast Cancer. 2017; 20:1–11.

17. Cendán JC, Coco D, Copeland EM 3rd. Accuracy of intraoperative frozen-section analysis of breast cancer lumpectomy-bed margins. J Am Coll Surg. 2005; 201:194–198.

18. Olson TP, Harter J, Muñoz A, Mahvi DM, Breslin T. Frozen section analysis for intraoperative margin assessment during breast-conserving surgery results in low rates of re-excision and local recurrence. Ann Surg Oncol. 2007; 14:2953–2960.

19. Weber WP, Engelberger S, Viehl CT, Zanetti-Dallenbach R, Kuster S, Dirnhofer S, et al. Accuracy of frozen section analysis versus specimen radiography during breast-conserving surgery for nonpalpable lesions. World J Surg. 2008; 32:2599–2606.

20. Fukamachi K, Ishida T, Usami S, Takeda M, Watanabe M, Sasano H, et al. Total-circumference intraoperative frozen section analysis reduces margin-positive rate in breast-conservation surgery. Jpn J Clin Oncol. 2010; 40:513–520.

21. Morrow M, Jagsi R, Alderman AK, Griggs JJ, Hawley ST, Hamilton AS, et al. Surgeon recommendations and receipt of mastectomy for treatment of breast cancer. JAMA. 2009; 302:1551–1556.

22. Jorns JM, Daignault S, Sabel MS, Wu AJ. Is intraoperative frozen section analysis of reexcision specimens of value in preventing reoperation in breast-conserving therapy? Am J Clin Pathol. 2014; 142:601–608.

23. Chen K, Zeng Y, Jia H, Jia W, Yang H, Rao N, et al. Clinical outcomes of breast-conserving surgery in patients using a modified method for cavity margin assessment. Ann Surg Oncol. 2012; 19:3386–3394.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download