Abstract
Purpose
Estrogen receptor (ER) and progesterone receptor (PR) have been used as indicators of endocrine system status since the mid-1970s in the clinical management of breast cancer. The predictive role of ER in endocrine therapy is undisputed, but the prognostic value of PR is still debated. The aim of this study was to investigate the clinical characteristics and prognosis of ER positive breast cancer with different PR expression levels.
Methods
A population cohort of 3,030 primary invasive ER positive breast cancer patients from a single cancer center underwent surgery and received adjuvant endocrine therapy from 2004 to 2010. The clinical and biological features of these patients with high PR-expressing tumors were compared with those of patients with low PR-expressing tumors. The follow-up data for disease-free survival (DFS), overall survival (OS), and breast cancer specific survival (BCSS) was obtained from 2,778 patients. Cox regression analysis was used to correlate biomarkers and tumor characteristics with DFS, OS, and BCSS.
Results
Tumors with low PR expression had more invasive pathological features and biological indexes than those with high PR expression. Low PR expression was an independent poor prognostic factor for DFS (p=0.014; hazard ratio [HR], 0.781; 95% confidence interval [CI], 0.641–0.950), OS (p=0.002; HR, 0.699; 95% CI, 0.560–0.873), and BCSS (p=0.005; HR, 0.714; 95% CI, 0.566–0.902). Furthermore, in low PR expressing tumors, patients who received chemotherapy had better DFS (p=0.002; HR, 0.449; 95% CI, 0.268–0.751), OS (p<0.001; HR, 0.341; 95% CI, 0.192–0.606), and BCSS (p<0.001; HR, 0.292; 95% CI, 0.156–0.549) than patients who did not received chemotherapy.
Recently, the incidence of breast cancer has increased drastically, and breast cancer has become the most malignant tumor among women worldwide. With the development of basic and clinical research, the treatment of breast cancer is currently combined therapy that includes surgery, radiotherapy, chemotherapy, endocrine therapy, and targeted therapy, and so forth. The therapeutic concept is also gradually evolving as new findings and therapeutic approaches become available. Endocrine therapy of breast cancer, especially of steroid-dependent breast cancer, has an irreplaceable advantage. The application of endocrine therapy ranges from salvage treatment of advanced breast cancer, to adjuvant therapy of early breast cancer, to prophylactic treatment of high-risk populations. Given its many benefits, it is hopeful that endocrine therapy could cure patients with breast cancer [1].
Although patients with estrogen receptor-positive (ER+) and progesterone receptor-positive (PR+) breast cancer can be treated with endocrine therapy, patients with different expression levels of hormone receptors have different sensitivity to endocrine therapy [23]. Some previous studies showed that the efficacy of tamoxifen for ER+ breast cancer with high PR expression was better than that of patients with low PR expression, in both the adjuvant setting and the treatment of metastatic breast cancer [45]. Data from the large ATAC (Arimidex, Tamoxifen, Alone or in Combination) adjuvant trial, a worldwide clinical trial comparing the efficacy of tamoxifen with that of the aromatase inhibitor (AI) anastrozole, showed that patients with ER+/PR+ tumors had a lower recurrence rate than those with ER+/PR− tumors (7.6% vs. 14.8%, respectively) [6]. In this study, we analyzed cases of patients who were diagnosed with primary breast cancer and treated with surgery in Breast Diseases Treatment Center of Hebei Province from 2004 to 2010. By summarizing their distribution and clinical characteristics, and performing a prognostic analysis of ER+ breast cancer with different PR expression profiles, we were able to identify a novel prognostic indicator, which could provide a future reference for the individualized treatment of breast cancer.
The patient population comprised a consecutive series of all patients with invasive and operable breast cancer who presented to a single regional cancer center between January 2004 and June 2010. The median age was 50 years (range, 22–88 years). In our trial, all patients who underwent curative surgery were discussed postoperatively at a multidisciplinary meeting, and appropriate adjuvant endocrine therapy was prescribed according to national guidelines. Adjuvant tamoxifen was administered to pre-menopausal patients, and adjuvant AI was administered to postmenopausal patients. A total of 1,263 premenopausal patients received tamoxifen therapy, 1,022 postmenopausal patients received AI, and 493 patients switched from tamoxifen to an AI after 2 or 3 years, because they became postmenopausal during the years of tamoxifen treatment. The patients' clinical information had been collected previously and stored in a database. Specific patient consent was not required because retrospective data from the medical records of patients who had previously signed information release documents were used in this study. This research was approved by the Ethics Committee of Fourth Hospital of Hebei Medical University (number: SCXK2014-0031).
A total of 4,632 women with invasive carcinoma were identified. The inclusion criteria were women who (1) had undergone mastectomy or breast conserving surgery; (2) were ER+ and had been administered standard endocrine therapy after surgery; (3) had no severe concomitant diseases; (4) had complete immunohistochemistry data including ER, PR, and human epidermal growth factor receptor 2 (HER2); and (5) had invasive breast carcinoma. Patients who were diagnosed with bilateral tumors or distant metastases at the preoperative work-up were excluded. At last, a total of 3,030 patients entered the final analysis (Figure 1).
All the immunohistochemistry slides for ER/PR/HER2 were reviewed again by two independent pathologists. Immunohistochemistry staining of 4-mm sections of formalin-fixed paraffin-embedded tissue was performed with anti-ER (clone SP1; Roche, Basel, Switzerland), anti-PR (clone 1E2; Roche), anti-HER2 (clone 4B5; Roche) primary monoclonal antibodies. The ER and PR were visualized and classified based on the percentage of positive cells according to a semi-quantitative system. The slides were scored by counting the number of positive cells regardless of the staining intensity versus the total number of cells and calculating the percentage of positive cells (positive cells/total cells in one field). The positivity of several fields was averaged and presented as the ratio of positive cells per field to total cells per field: <1%, negative (−); 1% to 25%, weakly positive (+); 26% to 50%, positive (++); and >50%, strongly positive (+++) [7]. For PR expression profiling, a cutoff point of 25% was used to distinguish between low-expression (≤25%) and high-expression tumors (>25%). This cutoff point value is similar to a consensus 20% suggested at the St. Gallen International Breast Cancer Conference in 2013 [8]. HER2 scoring was categorized as 0, 1+, 2+, or 3+. A score of 3+ was considered positive, a score of 2+ was considered equivocal, and a score of 0/1+ was defined as negative [9]. Tumors with a HER2 score of 2+ were usually further analyzed by fluorescence in situ hybridization (FISH). If not, these tumors were considered equivocal. The FISH results were considered positive, equivocal, or negative according to HER2 copy number or the HER2/chromosome 17 centromere (CEP17) ratio [10]. Any ambiguity in the reports was resolved by discussion with senior pathologists.
The follow-up, which involved a hospital visit, telephone, or mail interview, began from the first day after surgery. The starting point of the follow-up was the date of operation. The finishing point of the follow-up was June 30, 2014. For patients who died, the date and cause of death was recorded; all deaths not attributable to breast cancer were censored at the date of death. The primary outcome in this analysis was time to breast cancer death; time to death by any cause and time to recurrence (first episode, local and/or distant) were also analyzed. Accordingly, the primary endpoints were disease-free survival (DFS), overall survival (OS), and breast cancer specific survival (BCSS).
The distribution of steroid receptor status and other categorical variables were compared using the standard chi-square test. Survival curves were constructed with the Kaplan-Meier method. A multivariate Cox regression model was used to determine the association of clinical pathological characteristics with DFS, OS, and BCSS in patients treated with endocrine therapy. Hazard ratios (HRs) for DFS, OS, and BCSS were estimated using a Cox proportional hazards regression through a multivariate analysis. All the statistical tests were performed using SPSS Statistics version 19.0 (IBM Corp., Armonk, USA) with a two-sided significance level of 5%. Survival rates and HRs were presented with their 95% confidence intervals (CIs).
The clinicopathological features of 3,030 ER+ patients with invasive breast carcinoma were analyzed. The clinical and biologic tumor characteristics are summarized in Table 1. Overall, low PR expression occurred more often in the postmenopausal group (55.2% vs. 38.0%, p<0.001) and older age group (aged 50 and above, 62.9% vs. 46.9%, p<0.001). However, the tumor size was larger (>2 cm) in the low PR expression group than in the high PR expression group (57.8% vs. 51.8%, respectively; p=0.014). In addition, lymph node metastasis was more prevalent (≥4) in the low PR expression group than in the high PR expression group (24.8% vs. 19.7%, respectively; p=0.004). Finally, there was a higher percentage of invasive ductal carcinoma (67.5% vs. 63.8%, respectively; p=0.030) in the low PR expression group than in the high PR expression group. In terms of the association between PR expression level and molecular markers, compared with the high PR expression group, the low PR expression group exhibited higher HER2 expression (25.3% vs. 14.0%, respectively; p<0.001) (Table 1).
The follow-up data for DFS, BCSS, and OS were obtained for 2,778 patients (91.7%). During the follow-up period, 329 patients died; 28 patients died from causes not attributable to breast cancer, and 425 patients had recurrence and metastasis. The median follow-up period of all patients was 70 months (range, 42–125 months). Among all the patients who were involved in this study, 2,656 (96.1%) were treated with mastectomy and 122 (4.4%) were treated with breast-conserving surgery. 85.9% of them (2,387/2,778) received chemotherapy, and approximately one-third of the patients (1,047/2,778) received radiotherapy. In our study, few of the participants with HER2 positive tumors were treated with trastuzumab because of the expensive charge, so targeted therapy was not included in the analysis of prognosis.
Univariate analysis using the Kaplan-Meier method showed that the patients with low PR-expressing tumors had worse DFS, BCSS, and OS than those with high PR-expressing tumors. The log-rank test showed a significant difference in the time to relapse and death between the two groups, with a shorter DFS (p<0.001), OS (p<0.001), and BCSS (p<0.001) in the low PR expression group (Figure 2). To readdress these imbalances and investigate whether PR status was an independent and significant predictor of DFS, OS, and BCSS among patients who received adjuvant endocrine therapy, a multivariate analysis was performed comparing the high and low PR expression groups with adjustment of all significant variables. In general, the multivariate analyses demonstrated that high PR expression was still significantly associated with a better prognosis in terms of DFS (p=0.014; HR, 0.781; 95% CI, 0.641–0.950), OS (p=0.002; HR, 0.699; 95% CI, 0.560–0.873), and BCSS (p=0.005; HR, 0.714; 95% CI, 0.566–0.902) (Table 2). These results indicate that patients with low PR expression have a higher recurrence risk and a higher chance of death than patients with high PR expression. In this study, we determined that chemotherapy was also a protective factor of DFS (p=0.006; HR, 0.607; 95% CI, 0.425–0.867), OS (p<0.001; HR, 0.435; 95% CI, 0.293–0.646), and BCSS (p<0.001; HR, 0.418; 95% CI, 0.273–0.640) (Table 2). To determine whether the expression of PR was predictive of chemotherapy efficacy in breast carcinomas, a multivariate analysis was performed in both the high PR expression group and the low PR expression group. The results showed that patients with low PR expression who received chemotherapy had a better DFS (p=0.002; HR, 0.449; 95% CI, 0.268–0.751), OS (p<0.001; HR, 0.341; 95% CI, 0.192–0.606), and BCSS (p<0.001; HR, 0.292; 95% CI, 0.156–0.549) (Tables 3, 4, 5) than those who did not receive chemotherapy. However, in the high PR expression group, we found that chemotherapy was not associated with DFS (p=0.397; HR, 0.803; 95% CI, 0.483–1.335), OS (p=0.052; HR, 0.572; 95% CI, 0.325–1.005), or BCSS (p=0.098; HR, 0.599; 95% CI, 0.326–1.100) (Tables 3, 4, 5).
Breast cancer is one of the most common cancers among women worldwide [11]. The incidence of breast cancer continues to rise, and more than 15% of patients develop incurable disease [12]. It is important to identify those nonresponsive breast cancers and develop individualized therapies. Breast cancer gene expression profiling has gained significant advances in recent years. The combinatorial origin, the heterogeneity of malignant cells, and the variability of the host background create distinct molecular subgroups of tumors. However, patients with different molecular subgroups do not respond the same to endocrine therapy [13]. In addition, hormone receptor status can provide prognostic assessment for the effect of specific endocrine therapy for patients with breast cancer [14].
PR is an important molecular marker that can predict the prognosis of breast cancer and its response to endocrine therapy, especially in ER+ breast cancers. Progestogens have been shown to oppose estrogen-stimulated growth of an ER+/PR+ patient-derived xenograft in a previous study [15]. In addition, the expression of PR can hinder estrogen-mediated proliferation and ER transcriptional activity in ER+ breast cancer cells [16]. Furthermore, tumor metastasis could partly be inhibited by high levels of PR in early-stage disease, and administration of a progesterone injection prior to surgery can provide improved clinical benefit [17]. The above-mentioned results indicate that PR activation can have an anti-tumorigenic effect in the context of ER+ breast cancer. At the 2013 St. Gallen International Breast Cancer Conference, the expression level of PR was defined in the molecular classification criteria. The luminal A type was defined as ER+/PR+ with a PR cell number greater than 20% [8]. In our study, the cutoff value was 25%, which is similar to the consensus 20%. We investigated PR expression and its relationship to other clinical and pathological parameters and studied the expression of other molecular markers in patients with invasive breast cancer. Our results showed that patients with low PR expression were mostly aged 50 or above compared to patients with high PR expression. The association between age and PR expression level that we found in this study is consistent with Rakha et al.'s study [18]. The number of patients with low PR expression who were postmenopausal was significantly higher than that of patients with high PR expression; one previous report also showed this result [19].
In our study, we found that patients with low PR-expressing tumors had worse clinical and biologic characteristics than those with high PR-expressing tumors. The differences in tumor characteristics between the two groups might be related to hormone levels, which is currently widely recognized [20]. As the results indicate, compared to tumors with high PR expression, low PR-expressing tumors had a larger tumor size and more lymph node metastasis. Our findings also showed that low PR expression breast cancer is more likely than high PR expression breast cancer to be HER2-positive. Similar results were found in others research studies [220].
Adjuvant systemic therapy can significantly decrease breast cancer recurrence and mortality rates [21]. Endocrine therapy is recommended for most ER+ or PR+ patients due to its efficacy and favorable safety profile [21]. Some patients might receive endocrine therapy as their only adjuvant therapy. However, many unsolved questions prevent oncologists from selecting the appropriate endocrine therapeutic regimen for the distinct breast cancer subtypes. The role of ER expression level as a predictor of patients' response to endocrine treatment has been consistently recognized, but the role of PR status in the management of breast cancer remains controversial. To date, relatively few studies have been performed to find an association between PR status and prognosis of breast cancer. Even fewer studies exist of Chinese female patients with breast cancer. This study is a large and comprehensive evaluation of the prognosis of breast cancer in Chinese women with ER+ and different PR-expressing tumors. Furthermore, the relationship between PR status and chemotherapy effect was analyzed herein.
In our study, we found significant differences in DFS, BCSS, and OS through a multivariate analysis and each of these variables was an independent predictor of PR status, chemotherapy, lymph node metastasis, and factors, which is similar to results of previous studies [22232425]. Kakugawa et al. [26] suggested that decreased PR expression might lead to excessive proliferation of glandular cells, which can cause cancer and metastatic lesions. In our study, all of the subjects were ER+ and treated with any endocrine therapy. Several studies have also indicated that PR might be a marker for predicting the sensitivity of endocrine therapy [2]. Another study showed that PR is synthesized by tumor cells that are stimulated by estrogens through an interaction with ER [27]. Based on the above evidence, we can conclude that absence of PR expression might result in the loss of normal ER pathway function, which would account for the relative unresponsiveness to endocrine therapy.
Chemotherapy is also an important part of the treatment for breast cancer, and patients with different molecular subtypes have different chemosensitivity [28]. Few studies have been done to find an association between PR status and chemosensitivity. In our study, we found that patients with low PR-expressing tumors could benefit from chemotherapy. However, in patients belonging to the high PR expression group, the prognostic significance of chemotherapy was modest. In one study, HER2 was proven a useful marker to identify the chemosensitivity of breast cancer. Tumors with a higher expression of HER2 might be more sensitive to chemotherapy [29]. Furthermore, a previous study showed that PR loss correlates with HER2 overexpression in ER+ breast cancer [30]. The same results were obtained from our study. Taken together, we can infer that patients with low PR expression tumors might derive a greater benefit from adjuvant chemotherapy. Further studies are warranted to find possible methods to prove the results.
In clinical practice, it is very complex to use PR as a biological marker. Despite progress in understanding the structure and function of PR, it is still not widely used as either a predictive or prognostic marker in the treatment of cancer. When adjuvant treatment decisions are made with individual patients, especially when endocrine therapy alone or endocrine therapy combined with chemotherapy is considered, PR status might be an important additional consideration. Assessment of the PR status should be a mandatory part of assessing the prognosis of breast cancer patients.
Figures and Tables
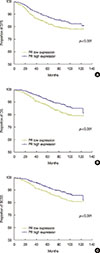 | Figure 2Univariate survival analysis of the estrogen receptor-positive (ER+)/progesterone receptor (PR) low expression patients and the ER+/PR high expression patients with disease-free survival (DFS), overall survival (OS) and breast cancer specific survival (BCSS). (A) DFS, (B) OS, and (C) BCSS. The figure show that low expression of PR expression was associated with significantly poorer DFS, OS, and BCSS in ER+ patients treated with any endocrine therapy. |
Table 1
The relationship between PR expression and clinicopathological characteristics

Table 2
Multivariate analyses of disease-free survival, overall survival, and breast cancer specific surviva

Table 3
Multivariate analyses of disease-free survival in low and high PR expression group

Table 4
Multivariate analyses of overall survival in low and high PR expression group

Table 5
Multivariate analyses of breast cancer specific survival in low and high PR expression group

Notes
References
1. Palmieri C, Patten DK, Januszewski A, Zucchini G, Howell SJ. Breast cancer: current and future endocrine therapies. Mol Cell Endocrinol. 2014; 382:695–723.

2. Bae SY, Kim S, Lee JH, Lee HC, Lee SK, Kil WH, et al. Poor prognosis of single hormone receptor-positive breast cancer: similar outcome as triple-negative breast cancer. BMC Cancer. 2015; 15:138.
3. Viale G, Regan MM, Maiorano E, Mastropasqua MG, Dell'Orto P, Rasmussen BB, et al. Prognostic and predictive value of centrally reviewed expression of estrogen and progesterone receptors in a randomized trial comparing letrozole and tamoxifen adjuvant therapy for postmenopausal early breast cancer: BIG 1-98. J Clin Oncol. 2007; 25:3846–3852.

4. Arpino G, Weiss H, Lee AV, Schiff R, De Placido S, Osborne CK, et al. Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst. 2005; 97:1254–1261.

5. Elledge RM, Green S, Pugh R, Allred DC, Clark GM, Hill J, et al. Estrogen receptor (ER) and progesterone receptor (PgR), by ligand-binding assay compared with ER, PgR and pS2, by immuno-histochemistry in predicting response to tamoxifen in metastatic breast cancer: a Southwest Oncology Group Study. Int J Cancer. 2000; 89:111–117.

6. Dowsett M, Cuzick J, Wale C, Howell T, Houghton J, Baum M. Retrospective analysis of time to recurrence in the ATAC trial according to hormone receptor status: an hypothesis-generating study. J Clin Oncol. 2005; 23:7512–7517.

7. Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract. 2010; 6:195–197.

8. Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol. 2013; 24:2206–2223.

9. Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013; 31:3997–4013.

10. Chao WR, Lee MY, Lin WL, Chen CK, Lin JC, Koo CL, et al. HER2 amplification and overexpression are significantly correlated in mucinous epithelial ovarian cancer. Hum Pathol. 2014; 45:810–816.

11. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55:74–108.

12. de Bono JS, Tolcher AW, Rowinsky EK. The future of cytotoxic therapy: selective cytotoxicity based on biology is the key. Breast Cancer Res. 2003; 5:154–159.

13. Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006; 295:2492–2502.

14. Li FY, Wu SG, Zhou J, Sun JY, Lin Q, Lin HX, et al. Prognostic value of Ki-67 in breast cancer patients with positive axillary lymph nodes: a retrospective cohort study. PLoS One. 2014; 9:e87264.

15. Kabos P, Finlay-Schultz J, Li C, Kline E, Finlayson C, Wisell J, et al. Patient-derived luminal breast cancer xenografts retain hormone receptor heterogeneity and help define unique estrogen-dependent gene signatures. Breast Cancer Res Treat. 2012; 135:415–432.

16. Zheng ZY, Bay BH, Aw SE, Lin VC. A novel antiestrogenic mechanism in progesterone receptor-transfected breast cancer cells. J Biol Chem. 2005; 280:17480–17487.

17. Mohammed H, Russell IA, Stark R, Rueda OM, Hickey TE, Tarulli GA, et al. Progesterone receptor modulates ERalpha action in breast cancer. Nature. 2015; 523:313–317.

18. Rakha EA, El-Sayed ME, Green AR, Paish EC, Powe DG, Gee J, et al. Biologic and clinical characteristics of breast cancer with single hormone receptor positive phenotype. J Clin Oncol. 2007; 25:4772–4778.

19. Liu S, Chia SK, Mehl E, Leung S, Rajput A, Cheang MC, et al. Progesterone receptor is a significant factor associated with clinical outcomes and effect of adjuvant tamoxifen therapy in breast cancer patients. Breast Cancer Res Treat. 2010; 119:53–61.

20. Huang HJ, Neven P, Drijkoningen M, Paridaens R, Wildiers H, Van Limbergen E, et al. Association between tumour characteristics and HER-2/neu by immunohistochemistry in 1362 women with primary operable breast cancer. J Clin Pathol. 2005; 58:611–616.

21. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Davies C, Godwin J, Gray R, Clarke M, Cutter D, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011; 378:771–784.

22. Xue C, Wang X, Peng R, Shi Y, Qin T, Liu D, et al. Distribution, clinicopathologic features and survival of breast cancer subtypes in Southern China. Cancer Sci. 2012; 103:1679–1687.

23. Rosa Mendoza ES, Moreno E, Caguioa PB. Predictors of early distant metastasis in women with breast cancer. J Cancer Res Clin Oncol. 2013; 139:645–652.

24. Xing P, Li JG, Jin F, Zhao TT, Liu Q, Dong HT, et al. Prognostic significance of body mass index in breast cancer patients with hormone receptor-positive tumours after curative surgery. Clin Invest Med. 2013; 36:E297–E305.

25. Dirier A, Burhanedtin-Zincircioglu S, Karadayi B, Isikdogan A, Aksu R. Characteristics and prognosis of breast cancer in younger women. J BUON. 2009; 14:619–623.
26. Kakugawa Y, Minami Y, Tateno H, Inoue H, Fujiya T. Relation of serum levels of estrogen and dehydroepiandrosterone sulfate to hormone receptor status among postmenopausal women with breast cancer. Breast Cancer. 2007; 14:269–276.

27. Arafah BM, Finegan HM, Roe J, Manni A, Pearson OH. Hormone dependency in N-nitrosomethylurea-induced rat mammary tumors. Endocrinology. 1982; 111:584–588.

28. Chen XS, Wu JY, Huang O, Chen CM, Wu J, Lu JS, et al. Molecular subtype can predict the response and outcome of Chinese locally advanced breast cancer patients treated with preoperative therapy. Oncol Rep. 2010; 23:1213–1220.

29. Yao L, Zhang J, Liu Y, Ouyang T, Li J, Wang T, et al. Association between HER2 status and response to neoadjuvant anthracycline followed by paclitaxel plus carboplatin chemotherapy without trastuzumab in breast cancer. Chin J Cancer Res. 2015; 27:553–561.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download