Abstract
Breast cancer is the second leading cause of death among women in North America. Glioblastoma is the most common primary malignant central nervous system tumor in adults. The majority of hereditary breast cancers are associated with deleterious mutations in the BRCA1 and BRCA2 genes. Although few case reports have described the incidence of glioblastoma in patients previously diagnosed with breast cancer, any association between BRCA2 mutations and glioblastoma has not been demonstrated to date. Herein, we report a woman who is a carrier of a familial BRCA2 mutation, and was previously diagnosed with triple-negative breast cancer (TNBC) and subsequently with a second primary TNBC and glioblastoma. Further investigation is required to define the possible relationship between these two aggressive malignances and the BRCA2 mutation, which might be critical for the proper management and treatment of this disease.
Breast cancer is the most common cancer as well as the second leading cause of death among women in North America [1], and glioblastoma is the most common and malignant among all primary brain tumors. Growing clinical and preclinical data suggest the existence of a genetic link between breast cancer and various other primary solid neoplasms [2]. Herein, we report a woman who is a carrier of a familial BRCA2 mutation, and was previously diagnosed with triple-negative breast cancer (TNBC) and subsequently with a second primary TNBC and glioblastoma.
The patient is a 48-year-old woman with a family history of breast cancer, who had presented with a lump in her left breast 10 years previously. A core biopsy showed poorly differentiated invasive ductal carcinoma negative for the estrogen and progesterone receptors, and human epidermal growth factor receptor 2. She underwent breast-conserving surgery (BCS) and sentinel lymph node biopsy (SLNB). The tumor was 1.5 cm, and lymph node involvement was absent. The patient was confirmed to have stage I (T1N0M0) cancer. She received four cycles of the doxorubicin and cyclophosphamide (AC) regimen under Cancer and Leukemia Group B 40101, followed by local radiation therapy.
The patient had a strong family history of breast cancer. Her mother died at the age of 59 years after being diagnosed with breast cancer at the age of 58 years. Her maternal grandmother was diagnosed with breast and ovarian cancer in her 50s and died at the age of 54 years. Information on the existence of other cancer types in her family was not available.
Nine years after her initial diagnosis, the patient developed a second primary mass in her right breast. The core biopsy showed poorly differentiated invasive ductal carcinoma of the breast. She underwent BCS and SLNB. Biomarker assessment revealed a triple-negative status. The tumor was 0.5 cm, and the final diagnosis was Stage I (T1aN0M0) TNBC. Metastasis was not detected in the SLNB.
The patient was initially diagnosed and treated at another institution, and for unknown reasons, she was not considered for genetic counseling and screening for BRCA mutations at the time. However, when she was examined at our cancer center, genetic screening for BRCA1 and BRCA2 mutations was performed, and a mutation (1963del5) was identified in the BRCA2 gene; however, systemic chemotherapy was not administered.
The risks and benefits of bilateral mastectomy were discussed with the patient and consent was obtained to perform the procedure. After 5 months, she also underwent prophylactic robotic hysterectomy and bilateral salpingo-oophorectomy. Eight months after the mastectomy, recurrence in the right chest wall and axillary recurrence were identified and resected. Pathological analysis confirmed recurrent triple-negative invasive ductal carcinoma with areas of high-grade ductal carcinoma in situ. The patient received four cycles of the docetaxel and cyclophosphamide (TC) regimen every 3 weeks. Within 1 month after the completion of chemotherapy, the patient developed symptoms of expressive aphasia with progressive headaches. Head computed tomography revealed a ring-enhancing mass in the left temporal occipital region with surrounding edema. Magnetic resonance imaging confirmed the presence of a mass measuring 3.6×3.5 cm (Figure 1). The patient underwent left sided temporo-occipital craniotomy and stereotactic volumetric resection of the left temporo-occipital brain lesion. The pathological findings were consistent with glioblastoma and showed fragments of a highly cellular malignant tumor with nuclear pleomorphism, vascular proliferation, pseudopalisading cells, and numerous mitotic figures, some of which were atypical. These areas strongly expressed glial fibrillary acidic protein and were negative for AE1/AE3 and CK7 (Figure 2) expression. Surgery was followed by concurrent chemoradiation therapy with a daily oral dose of 75 mg/m2 temozolomide. The patient then received 150 mg/m2 of adjuvant temozolomide daily for 5 days, every 28 days. The most recent brain magnetic resonance images obtained 1 year after resection of the glioblastoma did not show any evidence of disease.
Breast cancer is the most common cancer in women, constituting 29% of all cancers among women. Breast cancer is the second leading cause of death among women [1]. Several risk factors including genetic predisposition, environmental and hormonal factors, and obesity have been associated with the pathogenesis of breast cancer. The genetic mutations identified in most cases of breast cancer are sporadic rather than a consequence of inherited factors. Hereditary forms of breast cancer account for only 5% to 10% of all breast cancer cases irrespective of the fact that most women diagnosed with breast cancer have a positive family history of the disease.
The majority of hereditary breast cancers are associated with deleterious mutations in the BRCA1 and BRCA2 genes. BRCA1 is localized to chromosome 17q21 while BRCA2 is localized to chromosome 13q12-13. Among all breast cancer diagnoses, TNBC is prevalent in 15% to 20% of the cases. BRCA1 and BRCA2 mutations are associated with up to 15% of TNBCs. TNBC cases account for 70% of the breast cancers arising in carriers of BRCA1 mutations and for 16% to 23% of those arising in carriers of BRCA2 mutations [3]. In addition to breast and ovarian cancers, BRCA mutations are also associated with other cancers like male breast, pancreas, fallopian tube, and prostate cancers [2].
Glioblastoma is the most common primary malignancy of the central nervous system in adults, constituting 54% of all gliomas. Only one-third of patients with glioblastoma survive for 1 year and less than 5% live beyond 5 years, making glioblastoma the most lethal brain tumor.
Dubrow and Darefsky [4] observed an important similarity between glioblastoma and nonglioblastoma patients according to gender. For each subtype, the incidence rate was higher in men than in women. The men/women relative risk (RR) was statistically significant with a slightly higher RR for glioblastoma patients (1.6) than for nonglioblastoma patients (1.4).
Increasing evidence from clinical and preclinical data has suggested a genetic link between breast cancer and various other primary solid neoplasms. Glioblastomas have been associated with several genetic conditions such as Li-Fraumeni (p53 mutation) [5] and PTEN tumor suppressor gene mutations that also genetically predispose the patient to the development of breast cancer [6]. Although there are few case reports describing the observation of glioblastoma in patients previously diagnosed with breast cancer, no association between BRCA2 mutations and glioblastoma has been demonstrated [7].
Piccirilli et al. [8] reported 11 female patients treated for breast cancer with a diagnosis of glioblastoma. However, data on BRCA1 and BRCA2 mutations and biomarker analysis were not reported. Elmariah et al. [9] reported a multicentric glioblastoma in a patient with invasive breast cancer. Genetic screening showed the presence of a BRCA1 mutation. Reid et al. [10] suggested a correlation between BRCA2 886delGT and brain tumors. Biallelic BRCA2 mutations are found in Fanconi anemia group D1 patients. They reported two brothers who developed Wilms tumors and brain tumors, one of whom had developed a glioblastoma at the age of 9 years. Alter et al. [11] included the 868delTG mutation in an analysis of the clinical and molecular features associated with the BRCA2 mutations identified in Fanconi anemia group D1 patients. However, a definitive correlation between brain tumors and BRCA2 mutations could not be proven. Recently, Boukerroucha et al. [12] suggested that BRCA1 mutations are not instrumental for the development of glioblastoma. They reported two cases with tumor-suppressor protein expression maintained in the glioblastoma irrespective of a pathogenic germline BRCA1 mutation.
In a preclinical study involving the in vivo implantation of human glioblastoma cell lines into nude mice, an association between hormones, particularly estrogen, and the pathogenesis and overall survival associated with glial malignancies has been suggested [13]. In two population-based studies, a higher incidence of aggressive gliomas was observed in men than in women [1415]. McKinley et al. [15] observed a lower risk of developing glial tumors in premenopausal women, suggesting a possible protective effect of hormones. This effect decreased as women reached postmenopausal ages.
Herein, we presented a TNBC patient with a BRCA2 mutation, who subsequently developed glioblastoma. The incidence of TNBC and glioblastoma in our patient, who is a BRCA2 mutation carrier, made our case unique, as this combination was not previously reported. Our patient underwent bilateral oophorectomy, which could theoretically lead to a possible loss of the protective effect of hormones in developing glial malignancies. However, this relationship is very unlikely to have played a role in our patient owing to the close proximity (6 months) between the ovarian ablative procedure and the incidence of glioblastoma.
Whether a definite association exists between the BRCA2 genetic mutation and the development of two different solid malignancies in our patient, or whether it was a mere coincidence, remains unclear. In view of the poor clinical outcome and aggressive behavior of TNBC followed by glioblastoma, further investigation is required to better understand the possible relationship between these malignances and the BRCA2 mutation, which may be critical for the proper management and treatment of such cases.
Figures and Tables
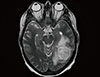 | Figure 1Magnetic resonance imaging. Magnetic resonance imaging of the brain shows a ring-enhancing mass in the left temporal occipital region with surrounding edema. |
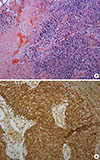 | Figure 2Histopathologic findings. (A) Histopathology shows tumor necrosis, hypercellularity, nuclear pleomorphism and vascular proliferation with cellular condensation (H&E stain, ×20). (B) Immunohistochemistry (IHC) image for glial fibrillary acidic protein (GFAP) shows positive staining in the malignant cells (and adjacent brain tissue) and absence of staining in the vessels (IHC for GFAP, ×20). |
References
2. Moran A, O'Hara C, Khan S, Shack L, Woodward E, Maher ER, et al. Risk of cancer other than breast or ovarian in individuals with BRCA1 and BRCA2 mutations. Fam Cancer. 2012; 11:235–242.

3. Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families: the Breast Cancer Linkage Consortium. Am J Hum Genet. 1998; 62:676–689.

4. Dubrow R, Darefsky AS. Demographic variation in incidence of adult glioma by subtype, United States, 1992-2007. BMC Cancer. 2011; 11:325.

5. Varley JM, Evans DG, Birch JM. Li-Fraumeni syndrome: a molecular and clinical review. Br J Cancer. 1997; 76:1–14.

6. Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997; 275:1943–1947.

7. Gastaut JL, Koeppel MC, Alliez B, Michel B, Gambarelli D, Martin PM. Triple tumor association: breast cancer, meningioma and glioblastoma. Rev Neurol (Paris). 1987; 143:753–758.
8. Piccirilli M, Salvati M, Bistazzoni S, Frati A, Brogna C, Giangaspero F, et al. Glioblastoma multiforme and breast cancer: report on 11 cases and clinico-pathological remarks. Tumori. 2005; 91:256–260.

9. Elmariah SB, Huse J, Mason B, Leroux P, Lustig RA. Multicentric glioblastoma multiforme in a patient with BRCA-1 invasive breast cancer. Breast J. 2006; 12:470–474.

10. Reid S, Renwick A, Seal S, Baskcomb L, Barfoot R, Jayatilake H, et al. Biallelic BRCA2 mutations are associated with multiple malignancies in childhood including familial Wilms tumour. J Med Genet. 2005; 42:147–151.

11. Alter BP, Rosenberg PS, Brody LC. Clinical and molecular features associated with biallelic mutations in FANCD1/BRCA2. J Med Genet. 2007; 44:1–9.

12. Boukerroucha M, Josse C, Segers K, El-Guendi S, Frères P, Jerusalem G, et al. BRCA1 germline mutation and glioblastoma development: report of cases. BMC Cancer. 2015; 15:181.

13. Plunkett RJ, Lis A, Barone TA, Fronckowiak MD, Greenberg SJ. Hormonal effects on glioblastoma multiforme in the nude rat model. J Neurosurg. 1999; 90:1072–1077.

14. Ohgaki H, Dessen P, Jourde B, Horstmann S, Nishikawa T, Di Patre PL, et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004; 64:6892–6899.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download