Abstract
Lymphedema is a condition characterized by tissue swelling caused by localized fluid retention. Advanced lymphedema is characterized by irreversible skin fibrosis (stage IIIb) and nonpitting edema, with leather-like skin, skin crypts, and ulcers with or without involvement of the toes (stage IVa and IVb, respectively). Recently, surgical treatment of advanced lymphedema has been a challenging reconstructive modality. Microvascular techniques such as lymphaticovenous anastomosis and vascularized lymph node flap transfer are effective for early stage lymphedema. In this study, we performed a two-stage operation in an advanced lymphedema patient. First, a debulking procedure was performed using liposuction. A vascularized free lymph node flap transfer was then conducted 10 weeks after the first operation. In this case, good results were obtained, with reduced circumferences in various parts of the upper extremity noted immediately postoperation.
Lymphedema is tissue swelling caused by localized fluid retention. Its natural course is chronic, progressive, and often destructive. It can either be congenital or acquired following trauma or surgery for cancer treatment that includes removal of the lymph nodes and radiotherapy [1].
The International Society of Lymphology classifies the severity of lymphedema into three stages. Karri et al. [2] reported a modified staging system, classifying the severity of lymphedema into four stages. According to their system, advanced lymphedema is characterized by irreversible skin fibrosis (stage IIIb) and nonpitting edema, with leather-like skin, skin crypts, and ulcers with or without involvement of the toes (stage IVa and IVb, respectively) [3].
Microvascular techniques such as lymphaticovenous anastomosis and vascularized lymph node flap transfer (VLNT) are effective for early stage lymphedema. Interestingly, liposuction, first used to treat lymphedema in 1989, has been shown to be an effective method of reducing limb volume and has good long-term results both cosmetically and functionally, In addition, liposuction has a low rate of complications and does not further disrupt the lymphatic system [4].
In this study, we performed a combined two-stage operation in an advanced lymphedema patient to achieve better results. First, a debulking procedure was performed using liposuction. VLNT was conducted 10 weeks after the first operation because there were no changes in the circumferences of various parts of the upper extremity after 2 months.
A 52-year-old woman had lymphedema in the right upper extremity caused by partial mastectomy with axillary lymph node dissection; she had under gone surgery and radiation therapy 10 years earlier for breast cancer. There was no improvement in symptoms following conservative treatment with rehabilitation and medication therapies. There was significant fibrosis in the upper extremity and pitting edema was not observed, indicating a reversible state of lymphedema. We defined this patient's lymphedema stage as IIIa (Figure 1).
Presurgical evaluation was done with lymphoscintigraphy, which indicated that there was no uptake in the right axillary lymph node. In addition, marked dermal back flow was noted, which is specific to lymphedema (Figure 2).
We planned a two-stage operation. First, a debulking operation using liposuction was arranged. Then, VLNT was planned for physiologic realignment of lymphatics.
Liposuction was performed with a cannulated catheter. During liposuction, fibrous septation was disrupted and 800 mL of fluid was drained. Immediately after liposuction, a garment was applied to prevent swelling. After 10 weeks, we performed a lymph node transfer using vascularized free flaps from the cervical region. We elevated the flap based around the transverse cervical artery as sigmoid shape in 1.5 cm above the clavicle. We then elevated the subplatysmal flap and identified the external jugular vein and omohyoid muscle between the sternocleidomastoid muscle and trapezius muscle. Next, we identified the transverse cervical artery, which is beneath the omohyoid muscle toward the anterior scalene muscle, and raised from the thyrocervical trunk. After flap elevation, we anastomosed the radial artery with the end-to-side method (Figure 3).
The severity of the lymphedema was evaluated by measuring the circumferences of various parts of the upper extremity. There were five points of measurement, including the wrist area, 10 cm distal from the cubital fossa, the cubital fossa, 10 cm proximal from the cubital fossa, and the axillary area.
There was improvement in the lymphedema condition following the operations. Ten weeks after the first liposuction operation, decreased circumferences ranging from 35.7% to 100% compared to those of the contralateral side were noted. Of note, we defined "%" as the ratio between the decreased circumferences and the difference between the normal side and affected side at their preoperative circumferences. One year after the second-stage lymph node transfer operation, all measurement points on the right upper limb except the wrist point had a smaller circumference than their preoperative measurement (Table 1, Figure 1).
The main mechanism of VLNT is re-establishment of lymphatic circulation in the transferred flap. In the postoperative period, healing of transplanted lymphatics to native lymphatics at the recipient site and removal of lymphatic fluid by a direct pumping mechanism may provide further fluid drainage [5]. In some patients, improvement in lymphedema swelling can be observed immediately in the hospital after VLNT, before the healing of the donor and native lymphatics. While the mechanism of improvement is unclear, the release of scar tissue in the previously operated and/or irradiated lymphatic bed has been postulated to account for this observation [6]. In addition, our patient showed immediate dramatic reduction of circumference length; 10 days after VLNT, the affected side arm had a smaller circumference than the contralateral normal side arm. This may have been caused by lymphatic absorption due to earlier scar tissue release caused by liposuction combined with strict arm elevation. We encouraged the patient to maintain a position with passive elevation of the arm 24 hours a day, using an elastic bandage sling attached to a standing pole.
Liposuction has been shown to remove circumferential subcutaneous fatty tissue in affected limbs. In addition, suction-assisted lipectomy has been demonstrated to maximally reduce excess solid volume remaining in lymphedema after the fluid component has been removed with conventional therapy. While effective in removing excess volume, debulking does not address the pathophysiology causing the lymphedema. Therefore, patients must continue postoperative compression to prevent reaccumulation of excess fluid, and additional postoperative therapy is required. Therefore, custom-fit compression garments are measured by the therapist and must be placed on the patient immediately postsurgery in the operating room.
It should be noted, however, that procedures that remove the fluid component of lymphedema, such as VLNT, are less likely to achieve the large reduction in excess volumes observed after liposuction. Rather, the procedures significantly decrease the postoperative need for compression garments and lymphedema therapy. Conversely, liposuction results in large volume reductions because it removes large amounts of solid fat and lymphatic material, but it does not address the ongoing lymphatic stasis and obstruction. Therefore, we have combined the liposuction and VLNT procedures in a two-staged approach to manage advanced staged lymphedema.
First, liposuction was performed to remove the solid components and reduce excess volume. After postoperative swelling stabilized, VLNT was used to improve lymphatic drainage and address subsequent fluid retention. Previously, this combined approach has resulted in volume reductions of over 83%, with compression garment use required only in the evenings and at night [7].
Furthermore, in this case report, we had to perform VLNT after liposuction to maintain superficial venous circulation. If we had performed VLNT before the liposuction, there would have been circulatory impairment caused by the liposuction procedure, which could have progressed to venous congestion. The compression garment applied immediately after liposuction also contributed to the prevention of venous retention.
VLNT requires microanastomosis, and there are three recipient sites available. The axillary area is usually operated on and irradiated, resulting in fibrotic changes, which make the dissection of recipient vessels more tedious. The anterior recurrent ulnar artery is sometimes very small. Therefore, the ulnar artery may be used with an end-to-side technique. For recipient vessels, the radial artery's dorsal branch and the cephalic vein are superficial and therefore easily dissected [8]. There is a hypothesis that VLNT may act by means of an internal pump and suction mechanism using pathways for lymphatic clearance of the lymphedematous limb. The "pump" mechanism is driven by the high-pressure inflow of the arterial anastomosis from the radial artery, which provides a strong hydrostatic force into the vascularized groin lymph node flap. The "suction" is continued by the large-caliber, superficially located, low-pressure venous drainage provided by the cephalic vein [9].
In this case, good results were obtained; immediately postoperation, the patient with advanced staged lymphedema had reduced circumferences in various parts of the upper extremity. However, there were limitations to this study. One limitation is that an increase in diameter was seen at each point between the immediate postoperative evaluation and the evaluation 1-year postoperation. We hypothesize that the increased circumferences after 1 year were the results of the stabilization of the grafted flap, rather than recurrence of lymphedema. Long-term follow-up observations would be needed to confirm this hypothesis. In addition, we did not evaluate the patient's satisfaction in psychological aspects of the study and physical activity; assessments of object findings are necessary in a later study.
Figures and Tables
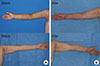 | Figure 1Preoperative evaluation and postoperative 1-year evaluation. (A) Preoperative status before vascularized lymph node transfer. (B) Postoperative 1-year evaluation. |
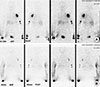 | Figure 2Lymphoscintigraphy evaluation. (A) Preoperative evaluation which seen in dermal back flow of right forearm. (B) Postoperative 1-year image shows increased lymphatic uptake in wrist area and reduced dermal back flow. |
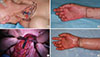 | Figure 3Flap elevation and anastomosis. (A) Preoperative surgical design on donor site of right cervical area. (B) Recipient design on right forearm area. (C) Anastomosis procedure of transverse cervical artery based lymph node flap. (D) Immediate postoperative state. |
Table 1
Clinical data of upper extremity circumferences during staged operations

References
1. Murdaca G, Cagnati P, Gulli R, Spanò F, Puppo F, Campisi C, et al. Current views on diagnostic approach and treatment of lymphedema. Am J Med. 2012; 125:134–140.

2. Karri V, Yang MC, Lee IJ, Chen SH, Hong JP, Xu ES, et al. Optimizing outcome of Charles procedure for chronic lower extremity lymphoedema. Ann Plast Surg. 2011; 66:393–402.

3. Sapountzis S, Ciudad P, Lim SY, Chilgar RM, Kiranantawat K, Nicoli F, et al. Modified Charles procedure and lymph node flap transfer for advanced lower extremity lymphedema. Microsurgery. 2014; 34:439–447.

4. O'Brien BM, Khazanchi RK, Kumar PA, Dvir E, Pederson WC. Liposuction in the treatment of lymphoedema: a preliminary report. Br J Plast Surg. 1989; 42:530–533.
5. Granzow JW, Soderberg JM, Kaji AH, Dauphine C. Review of current surgical treatments for lymphedema. Ann Surg Oncol. 2014; 21:1195–1201.

7. Granzow JW, Soderberg JM, Dauphine C. A novel two-stage surgical approach to treat chronic lymphedema. Breast J. 2014; 20:420–422.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download