Abstract
In patients with advanced breast cancer, most new calcifications detected on a mammogram after neoadjuvant chemotherapy are benign dystrophic calcifications, but this is not always observed. We present a patient with advanced breast cancer who had paradoxically increased malignant microcalcifications concomitant with primary tumor regression after undergoing neoadjuvant chemotherapy. After the neoadjuvant chemotherapy, the follow-up mammogram revealed that local, fine pleomorphic microcalcifications had markedly increased. Pathologically, these calcifications were ductal carcinoma in situ. We concluded that, in patients with breast cancer undergoing neoadjuvant chemotherapy, newly developed microcalcifications on follow-up mammograms should be carefully evaluated, and any suspicious malignant microcalcifications should be included in surgical excision planning.
The use of neoadjuvant chemotherapy for the treatment of patients with locally advanced breast cancer has increased. Neoadjuvant chemotherapy offers the advantage of reducing primary tumor size, thus allowing for the possibility of breast conservation surgery. Furthermore, evaluation of the tumor response to neoadjuvant chemotherapy also facilitates therapeutic regimen modification and the prediction of long-term outcomes [1].
Malignant calcifications may decrease after neoadjuvant chemotherapy, but usually do not completely disappear unless they are few in number [2]. In addition, persistent calcifications on follow-up mammograms after treatment do not necessarily indicate residual viable disease [2]. Residual microcalcifications after chemotherapy could be explained by the calcifications of necrotic residual tumor material, hematoma formation, or even fat necrosis [3]. To the best of our knowledge, only a few reports have described increased or newly developed malignant microcalcifications after neoadjuvant chemotherapy in patients with advanced breast cancer [1345].
We reported a patient with advanced breast cancer who, after undergoing neoadjuvant chemotherapy, presented with paradoxically increased malignant microcalcifications concomitant with primary tumor regression, which were confirmed histopathologically as ductal carcinoma in situ (DCIS).
A 36-year-old woman visited our institute for the treatment of newly diagnosed left breast cancer. She had a palpable lump for a month. A mammogram showed fine pleomorphic microcalcifications with segmental distribution in the left upper inner breast (Figure 1). No microcalcifications were observed in the left upper outer quadrant. Masses could not be detected on the mammogram because of the extremely dense fibroglandular breast tissue. A sonogram revealed multiple, irregular, hypoechoic infiltrative masses involving the upper inner and upper outer quadrants of the left breast (Figure 2). Contrast-enhanced magnetic resonance imaging (MRI) of the breast showed multicentric, nonmass enhancement lesions with segmental distribution and a heterogeneous internal enhancement pattern involving nearly all of the left upper inner and outer quadrants of the left breast (Figure 3). The largest lesion was 6 cm in diameter and 1 cm away from the nipple. The tumors initially showed rapid enhancement and delayed washout kinetics on dynamic MRI scans, which is typical of malignancy. No abnormal lymph node was detected on mammograms and sonograms. However, an abnormal lymph node with a maximum standard uptake value of 2.6 was noted on initial positron emission tomography-computed tomography (PET-CT). The stage was clinically assessed as T3N1M0.
The patient underwent four cycles of neoadjuvant chemotherapy with doxorubicin (60 mg/m2 intravenous injection) on day 1 plus cyclophosphamide (600 mg/m2 intravenous injection) on day 1, every 21 days, for 3 months. Follow-up imaging studies were performed and her mammograms revealed a marked increase in fine pleomorphic microcalcifications with regional distribution in both upper inner and upper outer quadrants of the left breast (Figure 4). However, a follow-up breast sonogram and MRI scan (Figures 5 and 6) showed partial regression of the size and extent of multifocal and multicentric tumors. An abnormal lymph node observed on initial PET-CT was normalized on follow-up PET-CT after completion of one cycle of neoadjuvant chemotherapy. The patient underwent a skin-sparing mastectomy with implant insertion. A histopathological examination revealed that most of the residual lesions were DCIS with microcalcifications, and invasive carcinoma cell nests were scattered among the DCIS lesions. On dissection of the axillary lymph node, we found that three of 10 lymph nodes from the left axilla had metastatic foci less than 1 mm in a diameter. The postoperative stage was ypT1N1miM0. Immunohistochemical analyses of the core biopsy specimen prior to neoadjuvant chemotherapy showed that both invasive ductal carcinoma and DCIS were estrogen receptor (ER) positive, progesterone receptor (PR) positive, and human epidermal growth factor receptor 2 (HER2) negative, and had a high Ki-67 labeling index (75%) (Figure 7). After chemotherapy, the surgical specimen of invasive ductal carcinoma was ER positive, PR positive, and HER2 negative and had a low Ki-67 index (5%–10%), while the DCIS was ER positive, PR negative, and HER2 positive and had a low Ki-67 index (5%–10%) (Figure 8).
In the present case, a patient with advanced breast cancer underwent neoadjuvant chemotherapy and showed a marked decrease in the size of multiple malignant masses, but also exhibited the development of suspicious local, fine pleomorphic malignant microcalcifications. These new calcifications were pathologically confirmed as extensive DCIS.
There are two types of calcification processes in breast pathology: a secretory process that occurs in benign lesions and a necrotic process that occurs in malignant lesions [3]. Breast cancers are polyclonal, and chemotherapy is effective only for certain subclones [4]. This results in scattered individual degenerative changes, with or without tumor cell apoptosis, throughout the tumor, concomitant with the development of dystrophic and psammomatous microcalcifications [4]. Tumor shrinkage in response to neoadjuvant chemotherapy occurs via two pathological mechanisms: multinucleated histiocytes phagocytose calcium deposits to decrease calcifications, whereas cancer cell necrosis can increase calcifications [6].
Mammography plays an important role in monitoring tumor response during neoadjuvant chemotherapy, especially if the tumor is associated with microcalcifications. Only mammography can enable an accurate assessment of the extent of microcalcifications, which is essential to determine the extent of excision. Several studies have reported that decreases in the size and density of the tumor mass on a mammogram were the most reliable indicators of treatment response [137]. By contrast, the appearance of and changes in microcalcifications associated with malignancy have been deemed inaccurate for evaluating tumor response [137].
Fadul et al. [4] showed the development of malignant-appearing microcalcifications after neoadjuvant chemotherapy in locally advanced breast cancer. These microcalcifications were histopathologically confirmed as microcalcifications of dystrophic and psammomatous types. Li et al. [5] reported an increased density of local microcalcifications after neoadjuvant chemotherapy in the absence of progressive disease. Vinnicombe et al. [8] reported the microcalcification status in 44 of 95 patients with breast cancer who underwent neoadjuvant chemotherapy. These microcalcifications decreased in four patients, were stable in 21, were more conspicuous in 15, and were newly developed in four. Adrada et al. [1] found that the extent of calcification after neoadjuvant chemotherapy had decreased in 35 of 106 patients with advanced breast cancer, but it had remained stable in 41 patients and had increased or was newly developed in 30 patients. They did not find any correlation between calcification changes and pathologic complete remission. However, none of these studies reported whether the newly developed calcifications were pathologically benign or malignant. In this case, new calcifications were pathologically confirmed as extensive DCIS. Our findings suggested that newly developed microcalcifications after chemotherapy are not always benign and dystrophic.
Adrada et al. [1] showed that ER-positive cancers had a significantly higher proportion of residual malignant calcifications after neoadjuvant chemotherapy compared to ER-negative cancers. Therefore, they suggested that residual calcifications related to incomplete excision might be less of a concern in cases of ER-negative breast cancer. Our case was an ER-positive cancer; however, we believe that newly developed, suspicious, malignant microcalcifications appearing on follow-up mammograms after treatment should be carefully assessed and confirmed histologically, regardless of the breast cancer subtype.
Gunia et al. [9] reported complete eradication of the noninvasive component despite the persistence of malignant-appearing microcalcifications in a patient receiving neoadjuvant chemotherapy plus trastuzumab. Our case showed an increase in malignant-appearing microcalcifications and the presence of an extensive intraductal component in the postoperative pathological result. We can report that the DCIS component in this case showed no response to the chemotherapy regimen, but the invasive cancer component revealed partial regression.
In conclusion, we present a patient with advanced breast cancer having paradoxically increased malignant microcalcifications accompanying primary tumor regression after neoadjuvant chemotherapy. The majority of new calcifications during chemotherapy are benign and dystrophic; however, as we demonstrated here, this is not always the case. Careful evaluations of microcalcifications through follow-up mammography are needed for patients who undergo neoadjuvant chemotherapy for locally advanced breast cancer. Any newly developed suspicious malignant microcalcifications in these patients should be excised during surgery.
Figures and Tables
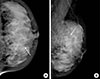 | Figure 1Initial mammograms. The cranio-caudal (A) and medio-lateraloblique (B) mammogram show fine pleomorphic microcalcifications in the left upper inner breast (arrows). However, masses are not detected on the mammogram because of the extremely dense fibroglandular breast tissue. |
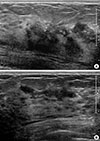 | Figure 2Initial ultrasonograms. The transverse (A) and longitudinal (B) ultrasonograms show an irregular hypoechoic infiltrative masses in the left upper inner and outer breast. Microcalcifications, however, are not detected on ultrasonography. |
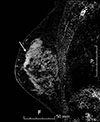 | Figure 3Initial magnetic resonance imaging (MRI) of the left breast. The early phase of the dynamic sagittal MRI with subtraction shows a nonmass enhancement lesion with segmental distribution and heterogeneous internal enhancement pattern in the left upper outer (arrow) and upper inner (not shown) quadrants. |
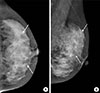 | Figure 4Follow-up left mammograms after neoadjuvant chemotherapy. The cranio-caudal (A) and medio-lateraloblique (B) mammograms show markedly increased number of fine pleomorphic microcalcifications (arrows), involving both upper inner and upper outer quadrants of the left breast. |
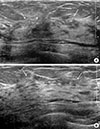 | Figure 5Follow-up ultrasonograms after neoadjuvant chemotherapy. The transverse (A) and longitudinal (B) ultrasonograms show a reduction in tumor size (arrows). |
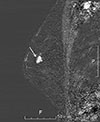 | Figure 6Follow-up breast magnetic resonance imaging (MRI) after chemotherapy. The early phase of the dynamic sagittal MRI with subtraction shows the decrease in the tumor extent in left upper outer quadrant (arrow). |
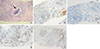 | Figure 7Histopathologic features of the core biopsy specimen of the left breast mass. (A) Invasive ductal carcinoma showing high histologic grade and small portion of ductal carcinoma in situ with necrosis and calcification (arrow) are observed (H&E stain). Invasive cancer tissue is luminal type B, showing positive reaction for estrogen receptor (B), low progesterone receptor expression (<20%) (C), high Ki-67 labelling index (>20%) (D), and 1+ reaction for human epidermal growth factor receptor 2 (E) (A–E, magnification, ×40). |
 | Figure 8Histopathologic features of the surgical specimen of the left mastectomy after neoadjuvant chemotherapy. (A) Small amount of residual invasive carcinoma tissues are scattered, and relatively conspicuous ductal carcinoma in situ lesions (H&E stain, ×40) (B) with necrosis and calcification (arrow) are seen (H&E stain, ×100). Invasive cancer tissue is changed to luminal type A after neoadjuvant therapy, showing positive reaction for estrogen receptor (C) and progesterone receptor (D), low Ki-67 labelling index (E), and 1+ reaction for human epidermal growth factor receptor 2 (F) (C-F, magnification, × 40). |
References
1. Adrada BE, Huo L, Lane DL, Arribas EM, Resetkova E, Yang W. Histopathologic correlation of residual mammographic microcalcifications after neoadjuvant chemotherapy for locally advanced breast cancer. Ann Surg Oncol. 2015; 22:1111–1117.

2. Segel MC, Paulus DD, Hortobagyi GN. Advanced primary breast cancer: assessment at mammography of response to induction chemotherapy. Radiology. 1988; 169:49–54.

3. Moskovic EC, Mansi JL, King DM, Murch CR, Smith IE. Mammography in the assessment of response to medical treatment of large primary breast cancer. Clin Radiol. 1993; 47:339–344.

4. Fadul D, Rapelyea J, Schwartz AM, Brem RF. Development of malignant breast microcalcifications after neoadjuvant chemotherapy in advanced breast cancer. Breast J. 2004; 10:141–145.

5. Li JJ, Chen C, Gu Y, Di G, Wu J, Liu G, et al. The role of mammographic calcification in the neoadjuvant therapy of breast cancer imaging evaluation. PLoS One. 2014; 9:e88853.

6. Esserman LE, d'Almeida M, Da Costa D, Gerson DM, Poppiti RJ Jr. Mammographic appearance of microcalcifications: can they change after neoadjuvant chemotherapy? Breast J. 2006; 12:86–87.

7. Londero V, Bazzocchi M, Del Frate C, Puglisi F, Di Loreto C, Francescutti G, et al. Locally advanced breast cancer: comparison of mammography, sonography and MR imaging in evaluation of residual disease in women receiving neoadjuvant chemotherapy. Eur Radiol. 2004; 14:1371–1379.

8. Vinnicombe SJ, MacVicar AD, Guy RL, Sloane JP, Powles TJ, Knee G, et al. Primary breast cancer: mammographic changes after neoadjuvant chemotherapy, with pathologic correlation. Radiology. 1996; 198:333–340.

9. Gunia SR, Patel MS, Mamounas EP. Pathologic complete response of HER-2 neu-positive invasive ductal carcinoma and ductal carcinoma in situ following neoadjuvant chemotherapy plus trastuzumab: a case report and review of literature. Case Rep Surg. 2012; 2012:454273.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download