Abstract
In acute leukemia, leukemic infiltration of the breast is extremely rare. We report a case of biphenotypic acute leukemia (BAL) that presented as a breast mass. A 30-year-old woman presented with a 4-month history of a right breast mass with nipple discharge and easy fatigue. She had received chemotherapy and peripheral blood stem cell transplantation for BAL and had been in complete remission for the last 2 years. Core needle biopsy of the breast mass revealed monomorphous infiltrates of blast cells with round nuclei and fine chromatin, consistent with leukemic infiltration. Subsequent bone marrow biopsy showed diffuse infiltration of immature cells. However, bone marrow karyotyping showed 46, XY, suggesting complete engraftment of transplanted donor cells. This is the report of BAL recurring as a breast mass. In the differential diagnosis of a breast mass, extramedullary relapse should be considered when the patient has a history of leukemia.
Relapse of acute leukemia presenting as extramedullary leukemic infiltration is uncommon [1]. Although any organ may be infiltrated by leukemic cells, breast infiltration is extremely rare. Breast infiltration as the initial presentation or relapse of leukemia can be mistaken for a benign breast mass or primary breast cancer. To my knowledge, there has been no previous case report of leukemic infiltration of the breast by biphenotypic acute leukemia (BAL). Most case reports have described extramedullary leukemic infiltration by acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL). Here I present the case of a patient with extramedullary infiltration of the breast as a presentation of BAL relapse and review the literature.
In March 2014, a 28-year-old Korean woman, at 24 weeks of gestation, presented with easy fatigue and an abnormal blood test. A complete blood count revealed a total white blood cell (WBC) count of 2.69×109/L with 12% immature cells, a hemoglobin level of 6.8 mg/dL, and a platelet count of 81×109/L. A bone marrow biopsy showed 78% blasts with various size nucleoli, fine chromatin, and basophilic cytoplasm. Immunophenotyping of the bone marrow revealed that the patient was positive for the following: CD7, CD13, CD19, CD33, CD117, cCD79a, HLA-DR, and terminal deoxynucleotidyl transferase (TdT); and negative for the following: CD2, CD3, CD5, CD20, cCD22, CD10, CD14, CD61, and myeloperoxidase. This result showed coexpression of markers for B lymphoid (CD19 and cCD79a) and myeloid lineage (CD117, CD13, and CD33), based on which a diagnosis of BAL was confirmed. After a Cesarean delivery at 28 weeks of gestation, the patient was treated with induction and consolidation therapy from May 2014 to September 2014. The induction regimen included daunorubicin 90 mg/m2 on days 1 to 3, vincristine 2 mg/m2 on days 1 and 8, prednisolone 60 mg/m2 on days 1 to 14, L-asparaginase 4,000 units/m2 on days 17 to 28, and intrathecal methotrexate 15 mg on days 2 and. After finishing induction therapy, three cycles of consolidation therapy were administered. For the first and third cycles, she was treated with daunorubicin 45 mg/m2 on days 1 and 2, vincristine 1.4 mg/m2 on days 1 and 8, prednisolone 60 mg/m2 on days 1 to 14, and L-asparaginase 4,000 units/m2 on days 1 to 7. For the second cycle, she received cytarabine 2,000 mg/m2 on days 1 to 4 and etoposide 150 mg/m2 on days 1 to 4. Methotrexate was used during the third cycle of consolidation therapy (1,660 mg/m2 on days 1 and 15; 720 mg/m2 on days 2 and 16). In November 2014, she successfully underwent allogenic peripheral blood stem cell transplantation from a male donor.
There was no evidence of relapse for 23 months after the stem cell transplantation. In September 2016, the patient presented with easy fatigue, a palpable right breast mass, and nipple discharge, which had developed 4 months prior. She had no history of bleeding, weight loss, or dizziness over the previous few months. On physical examination, a 6 cm×6 cm firm, irregular, nontender mass was palpated in the upper inner area of her right breast. Mammography showed extremely dense breast tissue but no well-defined mass (Figure 1A). However, breast ultrasonography revealed 5.7×2.1×5.9 cm and 3.0×1.4×2.5 cm masses with partly indistinct margins and a heterogeneous echoic pattern in her right breast (Figure 1B). A complete blood count showed a total WBC count of 7.41×109/L, a hemoglobin level of 14.3 mg/dL, and a platelet count of 151×109/L. A core needle biopsy (CNB) was performed on the 2 o’clock direction mass in the right breast. Microscopic examination revealed monomorphous infiltrates of blast cells with round nuclei and fine chromatin (Figure 2A). The immunohistochemical (IHC) staining showed that the majority of blast cells were positive for CD34 and CD117 (Figure 2B and C). The blast cells were not stained for myeloperoxidase (Figure not shown). A bone marrow karyotyping demonstrated 46, XY and no recipient XX cells, which suggested complete engraftment of transplanted donor cells. Bone marrow differential counting showed 3.8% blasts and a 2.1:1 ratio of myeloid to erythroid precursors, which were within normal ranges. However, a bone marrow biopsy showed diffuse infiltration of immature cells with irregular nucleoli and a markedly decreased number of normal hematopoietic cells. These findings were consistent with leukemic infiltration.
Infiltration of the breast by acute leukemia is rare, regardless of whether it is found at initial presentation or at relapse after treatment. Viadana et al. [2] reviewed autopsy data of 503 patients with leukemia. Of 235 patients with AML, only four had involvement of the breast (1.7%). Although few cases have been described, breast infiltration seems more likely to occur in patients with AML rather than those with ALL [34]. The mechanism of extramedullary infiltration is unclear. In studies of extramedullary AML, the expression of adhesion molecules such as CD56 was associated with extramedullary granulocytic sarcoma, which occurred in 3% to 7% of AML cases [56].
Patients with leukemic infiltration of the breast usually present with a palpable breast mass that mimics a benign breast lesion, such as a fibroadenoma. Mammography and breast ultrasonography are commonly used to detect breast lesions. On mammography, findings are variable and can be unremarkable with diffusely dense breast parenchyma. On ultrasonography, no definitive imaging pattern suggesting leukemic infiltration of the breast has been described because sonographic features of tumors vary from hypoechoic to hyperechoic masses [78]. Magnetic resonance imaging (MRI) may be another diagnostic tool, especially in patients with dense glandular tissue, breast implants, or pregnancy. MRI features include T2 hyperintensity and rapid ring-enhancement of the lesions [9].
For a final diagnosis of leukemic infiltration of the breast, tissue evaluation must be performed. A CNB under ultrasono-graphy guidance is an acceptable and safe method to establish the diagnosis and avoid unnecessary surgical biopsy. The cytologic features of breast infiltration of ALL include dispersed monomorphic blastic cells with variable size, high ratio of nuclear to cytoplasmic, round or convoluted nuclei with dispersed chromatin, and scanty cytoplasm [10]. Our case showed monomorphic blast cells with round nuclei and fine chromatin. In the initial bone marrow biopsy of our case, CD7, CD13, CD19, CD33, CD117, cCD79a, HLA-DR, and TdT were positive, indicating expression of both myeloid cell and lymphoid cell markers. In the biopsy of breast leukemic infiltration, CD34 and CD117 were diffusely positive with IHC staining. Based on literature reviews, leukemic infiltration of the breast by acute leukemia has occurred in patients with AML and ALL. However, to the best of our knowledge, this is the first report of leukemic infiltration of the breast by BAL.
The treatment of extramedullary relapse after bone marrow transplantation remains controversial. Our patient received reinduction therapy, and many patients may benefit from chemotherapy [11]. Radiation therapy may be helpful to treat an isolated lesion with systemic therapy [12]. The role of surgical intervention for breast leukemic infiltration is much less clear. Most experts believe that there is no role of surgery for breast leukemic infiltration because there is no evidence of a clinical benefit of surgery for breast lesions, and surgery may delay the initiation of systemic therapy [13].
In conclusion, breast infiltration by BAL is extremely rare. It should be considered in the differential diagnosis of breast masses, especially when there is a history of acute leukemia. Mammography and breast ultrasonography may be useful to detect breast nodules. With CNB and IHC staining for leukemia markers, a definitive diagnosis can be established while avoiding unnecessary breast surgery, thus enabling early initiation of systemic therapy.
Figures and Tables
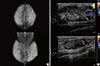 | Figure 1Radiologic findings for right breast mass. (A) Mammography showed extremely dense breast tissue but no well-defined mass. (B) Breast ultrasonography revealed a 5.7×2.1×5.9 cm and 3.0×1.4×2.5 cm masses with partly indistinct margins and heterogeneous echoic pattern in her right breast 2 o'clock direction and 8 o'clock direction, respectively. |
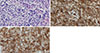 | Figure 2Microscopic findings of right breast mass. (A) Breast biopsy specimen shows monomorphous infiltrates of blast cells with round nuclei and fine chromatin (H&E stain, ×400). (B) Immunohistochemical staining for CD34, showing staining of the majority of the blast cells (×400). (C) Immunohistochemical staining for CD117, showing diffuse positive staining of the blast cells (×400). |
References
1. Jensen IM, Jensen PD, Ellegård J, Bastrup-Madsen P, Hokland P. Extramedullary manifestations among adult patients with acute lymphoblastic leukemia (ALL). Ugeskr Laeger. 1991; 153:1125–1129.
2. Viadana E, Bross ID, Pickren JW. An autopsy study of the metastatic patterns of human leukemias. Oncology. 1978; 35:87–96.

3. Khoury NJ, Hanna Al-Kass FM, Jaafar HN, Taher AT, Shamseddine AI. Bilateral breast involvement in acute myelogenous leukemia. Eur Radiol. 2000; 10:1031.

4. Yang WT, Muttarak M, Ho LW. Nonmammary malignancies of the breast: ultrasound, CT, and MRI. Semin Ultrasound CT MR. 2000; 21:375–394.

5. Khan MY, Hussein KK, Walter MG, Hasan MK, Kern W, Kharfan-Dabaja MA. Granulocytic sarcoma presenting with malignant anasarca in a patient with secondary acute myeloid leukemia. Int J Hematol. 2004; 79:250–252.

6. Karbasian-Esfahani M, Wiernik PH, Yeddu M, Abebe L. Leukemic infiltration of the breast in acute lymphocytic leukemia (ALL). Hematology. 2008; 13:101–106.

7. Memis A, Killi R, Orguc S, Ustün EE. Bilateral breast involvement in acute lymphoblastic leukemia: color Doppler sonography findings. AJR Am J Roentgenol. 1995; 165:1011.

8. Likaki-Karatza E, Mpadra FA, Karamouzis MV, Ravazoula P, Koukouras D, Margariti S, et al. Acute lymphoblastic leukemia relapse in the breast diagnosed with gray-scale and color Doppler sonography. J Clin Ultrasound. 2002; 30:552–556.

9. Basara I, Orguc S. Giant breast involvement in acute lymphoblastic leukemia: MRI findings. J Breast Cancer. 2012; 15:258–260.

10. Besina S, Rasool Z, Samoon N, Akhtar OS. Acute lymphoblastic leukemia presenting as a breast lump: a report of two cases. J Cytol. 2013; 30:201–203.

11. Yoo SW, Chung EJ, Kim SY, Ko JH, Baek HS, Lee HJ, et al. Multiple extramedullary relapses without bone marrow involvement after second allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia. Pediatr Transplant. 2012; 16:E125–E129.

12. Belasco JB, Goldwein JW, Simms S, Griffin G, D'Angio G, Lange B. Hypofractionated moderate dose radiation, intrathecal chemotherapy, and repetitive reinduction/reconsolidation systemic therapy for central nervous system relapse of acute lymphoblastic leukemia in children. Med Pediatr Oncol. 2000; 34:125–131.

13. Talwar V, Sreedharan PS, Warrier NK, Guhan , Ramanan SG, Sagar TG. Acute lymphoblastic leukemia presenting as breast mass. J Assoc Physicians India. 2000; 48:1212–1213.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download