Abstract
Purpose
The purpose of this study was to assess the value of adding digital breast tomosynthesis (DBT) to full-field digital mammography (FFDM) in the diagnostic workup of breast cancer and to determine which lesion variables affect cancer detectability in the combined modality.
Methods
Between March and May 2012, paired FFDM and DBT images were obtained from 203 women as part of a diagnostic workup for breast cancer. Images from FFDM alone, DBT alone, and DBT combined with FFDM were reviewed in separate sessions by six blinded readers. Jackknife alternative free-response receiver operating characteristic (JAFROC) figure of merit (FOM), sensitivity, and specificity were compared between the modalities. Lesion characteristics affecting the cancer detection rate when using the combined modality were also analyzed.
Results
Among the 203 women, 126 women had a total of 129 malignancies and 77 women had total of 77 benign lesions. The overall JAFROC FOM of the combined modality was higher than that of FFDM alone (0.827 vs. 0.775, p<0.001) and that of DBT alone was higher than that of FFDM alone (0.807 vs. 0.775, p=0.027). The overall sensitivity of the combined modality was higher than that of FFDM alone (80.0% vs. 73.2%, p<0.001) and that of DBT alone was higher than that of FFDM alone (78.3% vs. 73.2%, p=0.007). Compared to FFDM alone, the combined modality detected an additional 48 cancers. Using the combined modality, the presence of masses or microcalcifications was significantly associated with the cancer detection rate (p<0.001).
Conclusion
The combination of DBT with FFDM results in a higher diagnostic yield than FFDM alone. Additionally, DBT alone performs better than FFDM alone. However, even when DBT is combined with FFDM, breast cancers with no discernible masses and those lacking calcifications are difficult to detect.
Mammography, which is a mainstay of breast cancer detection, has long been used to reduce morbidity and mortality in patients with breast cancer [123]. Compared to film mammography, full-field digital mammography (FFDM) has significantly improved the performance of radiologists, particularly when interpreting examinations performed on women with dense breast tissue [4]. However, two-dimensional (2D) FFDM has been shown to be limited in the visualization of overlapping dense fibroglandular breast tissue, which can ultimately reduce the conspicuity of breast cancers and make normal structures appear abnormal [56].
In an effort to overcome the limitations of 2D FFDM, digital breast tomosynthesis (DBT), which is a new three-dimensional (3D) radiographic technique, has recently been introduced. DBT allows more precise evaluation of lesions through better differentiation of overlapping tissue [7891011]. This modality has also demonstrated potential advantages in the evaluation of masses, areas of architectural distortion, and asymmetries compared with conventional 2D mammography. Indeed, a reduction in recall rate and gain in diagnostic accuracy with the use of DBT in conjunction with FFDM, or by itself, have been demonstrated in several multicenter and screening trials [12131415161718]. Superior performance with respect to detection, localization, and characterization of multiple abnormalities per examination were also noted using this combined mode versus FFDM alone [19]. However, the factors that may affect detectability in DBT combined with FFDM have not been evaluated, and there is only limited data available on the type of lesion that can be easily detected or not detected in the clinical setting even with DBT [141520]. We surmised that investigating the reasons for the non-detection of occult cancers and obtaining information about the benefits and limitations afforded by DBT in the diagnostic setting would prove valuable to radiologists. Therefore, the purpose of this study was to retrospectively evaluate the value of adding DBT to FFDM, and to determine which lesion variables may affect the cancer detectability of the combined modality of DBT and FFDM.
This study was approved by the Institutional Review Board of our hospital (IRB number: 2012-0047), and written informed consent for DBT was obtained from all participants.
From March to May of 2012, we prospectively performed FFDM and DBT on women who were referred to our institution with clinical signs and symptoms of breast cancers, or who had suspicious findings on screening mammography or ultrasonography (US). Of the 219 consecutive women who received FFDM and DBT, 16 patients were excluded who either had a surgical clip in the breast or had a history of vacuum assisted breast biopsy as either could provide information regarding lesion location. As a result, a total of 203 women were enrolled in this study. The mean age of the patients was 49.8±11.2 years (range, 22–78 years). Among these patients, 107 (52.7%, 107/203) were asymptomatic but exhibited abnormalities on screening mammography (n=68) or US (n=39). Abnormal findings on FFDM in 68 patients included: dense breast tissue in three, mass in 20, calcification in 20, asymmetry in 17, distortion in one, mass with calcification in one, mass with distortion in one, asymmetry with calcification in three, calcification and distortion in one, and left breast mass and right breast calcification in one. Thirty-nine patients who had an abnormality detected on US showed either a benign mass, benign calcification, asymmetry, or no abnormal findings on mammography. The other 96 patients (47.3%, 96/203) showed clinical signs and symptoms of breast lesions including palpable lumps (n=85), pain (n=6), nipple discharge (n=3), nipple retraction (n=1), and Paget's disease (n=1). Of the 203 patients in this study, 98 patients had previously been evaluated elsewhere for known breast cancer. Clinical and histopathologic data were obtained from the electronic medical records and standard histopathologic report.
Breast composition of the patients according to the Breast Imaging Reporting and Data System (BI-RADS) recorded during the initial clinical interpretation were as follows: almost entirely fatty tissue (BI-RADS composition of a) (n=8, 3.9%), scattered fibroglandular densities (BI-RADS composition of b) (n=38, 18.7%), heterogeneously dense tissue (BI-RADS composition of c) (n=100, 49.3%), and extremely dense tissue (BIRADS composition of d) (n=57, 28.1%). For statistical analysis, breast density was dichotomized as fatty (BIRADS density categories a and b) versus dense (BIRADS density categories c and d).
FFDM and DBT images of both breasts in the craniocaudal (CC) and mediolateral oblique (MLO) positions were obtained using a commercially available device (Selenia Dimensions System; Hologic, Bedford, USA) in the combo mode by one dedicated technologist. DBT acquisition was performed with the X-ray tube moving over a 15° arc, while the breast remained compressed in the conventional manner, allowing a series of ultra-low dose images. After acquisition, data from the projection images were combined to create a full 3D-image set of 1 mm slices of the breast. Radiation doses used for DBT with FFDM were approximately twice that used for routine FFDM alone. Mean glandular dose per examination was 1.71±0.51 mGy for each CC view and 1.65±0.61 mGy for each MLO view for DBT, and 1.68±0.68 mGy for each CC view and 1.62±0.75 mGy for each MLO view for FFDM.
Six radiologists who specialize in breast imaging and with experience ranging from 2 to 15 years in reading mammograms participated in this reader study. The readers were blinded to the patient history, clinical reports, histopathologic information, and results obtained with other modalities. Prior to the study, each reader received additional training using DBT images from 50 known cases (35 cancers and 15 benign lesions). Those images had been obtained at our institution for other research purposes and were not included in this particular study. After training was completed, the readers independently interpreted two-view FFDM alone, two-view DBT alone, and combined FFDM and DBT datasets. To minimize learning bias, a 12-week interval elapsed between each reading session. The first and second reading sessions each consisted of half of the two-view FFDM alone and half of the two-view DBT alone images, randomized, and presented in sequential order. The third reading session consisted of all of the images from the combined FFDM and DBT dataset. The readers were instructed to detect the tumors, if any, and to record the location of each lesion. Identifiers were used to confirm that the readers had correctly identified a cancer. After identifying the lesions, the readers provided a number from 0 to 100 to rate the likelihood of malignancy and designated a forced BI-RADS category of 1, 2, 3, 4 or 5 to indicate the most likely outcome based on the appearance of the lesion. Cases assigned to BI-RADS categories of 1, 2, or 3 were considered to be normal or benign, and those assigned to BI-RADS categories of 4 or 5 were considered to be abnormal.
After the independent reader study, two dedicated breast radiologists who did not participate in the first part of the study reviewed the FFDM and DBT images together using the reference location of cancers identified on US or magnetic resonance imaging. They determined mammographic lesion size, distance from the nipple to the lesion, lesion location quadrant, lesion type, and breast composition based on BIRADS classification. The lesion type was coded separately as to whether it had the characteristics of mass, microcalcification, asymmetry, or architectural distortion.
Results of histopathologic analysis performed on samples obtained via biopsy or surgery, if available, or a follow-up period of more than 2 years in those cases not biopsied were used as the reference standard. Cases with malignant biopsy results were considered positive. Cases with benign biopsy results or cases that did not undergo biopsy but did not have evidence of malignancy during 2 years of follow-up imaging were considered negative. Lesion matching between FFDM/DBT and histopathology was performed off-site by two radiologists provided with the histopathologic reports and the standardized templates used in the image review. Surgical histopathology and core specimens were reviewed by one pathologist with 25 years of experience in breast histopathology.
The clinical, radiological, and histopathological findings of the subsequent surgical specimens, if available, for the 206 lesions were reviewed. Diagnostic performances of each reader with the three modalities were compared using jackknife alternative free-response receiver operating characteristic (JAFROC) curves constructed from the likelihood of malignancy rating for FFMD alone, DBT alone, and combined FFDM and DBT images. Diagnostic sensitivities and specificities were calculated from BI-RADS scores. The McNemar test was used to compare individual sensitivity and specificity for each modality. JAFROC analysis was used to analyze free-response receiver operating characteristic (FROC) data acquired using the multiple-reader multiple-case protocol. JAFROC figure of merit (FOM) was adjusted for the clustering effect of multiple readers as a random factor. Analysis of overall sensitivity and specificity across all readers for each modality was performed using a generalized estimating equation with an exchangeable correlation structure.
To identify lesion variables which could affect the cancer detectability of the combined modality, associations between detectability and the patient's clinical factors (age and symptoms) as well as lesion variables (mammographic lesion size, distance from the nipple to the lesion, lesion location quadrant, lesion type, breast composition based on BI-RADS classification, and histopathology) were assessed using ordinal logistic regression analysis. The detectability was assumed to be proportional to the number of readers who detected the lesions. To determine the most important factors affecting the detection performance of the combined modality, the association between the patient's clinical factors, lesion variables, and the number of readers who detected the lesions were examined using a cumulative logit model based on multinomial distribution with the proportional odds assumption. The proportional odds assumption was tested, and multicollinearity among the predictors was controlled through stepwise variable selection. All statistical analyses were performed using SPSS version 19.0 (IBM Corp., Armonk, USA), SAS version 9.2 (SAS Institute Inc., Cary, USA), and JAFROC (http://www.devchakraborty.com); p-values <0.05 were indicative of statistical significance.
Of the 206 lesions identified in 203 women, 129 (63.0%) were malignant and 77 (37.0%) were benign. Histopathologic confirmation was available for 188 lesions, this included all lesions identified as carcinoma (n=129) as well as 59 benign lesions. The duration of US follow-up for lesions with benign histologic findings was 22.5 to 23.8 months (median, 23.1 months); lesion stability was confirmed in all cases. Eighteen lesions were presumed to be benign without histologic confirmation, and there was no evidence of malignancy during the follow-up period of more than 2 years.
The mean tumor size of the invasive cancers was 2.1±1.3 cm (range, 0.1–7.7 cm), and that of ductal carcinoma in situ (DCIS) was 3.1±1.8 cm (range, 0.5–6.0 cm). Malignant histologic findings included 104 infiltrating ductal carcinomas (IDCs) with or without DCIS, 3 mucinous carcinomas, 2 invasive papillary carcinomas, 1 medullary carcinoma, 2 invasive lobular carcinomas, 1 invasive mixed ductal and lobular carcinoma, and 16 DCIS. Benign lesions which were histologically confirmed included fibrocystic change (n=18), fibroadenoma (n=28), intraductal papilloma (n=5), columnar cell change (n=2), sclerosing adenosis (n=1), radial scar (n=1), phyllodes tumor (n=1), benign mammopathy (n=1), atypical lobular hyperplasia (n=1), and lobular carcinoma in situ (n=1).
JAFROC FOMs using the likelihood of malignancy rating (%) for FFDM alone, DBT alone, and the combined modality for each reader as well as the overall FOM for all readers are listed in Table 1. The FOM of the combined modality was significantly higher than that of FFDM alone in four readers (p=0.045 for reader A; p=0.001 for reader B; p=0.002 for reader D; and p=0.003 for reader F). For readers B and D, the FOM of DBT alone was also higher than that of FFDM alone (p=0.014 for reader B; p=0.026 for reader D). For reader F, the FOM of the combined modality was higher than that of DBT alone (p=0.008). The overall FOM of combined DBT and FFDM was 0.827, which was significantly higher than that of FFDM alone (0.775) (p<0.001). The overall FOM of DBT alone was also higher than that of FFDM alone (0.807 vs. 0.775, p=0.027). There was no statistically significant difference between the FOM for the combined modality and that of DBT alone (p=0.144).
The sensitivity and specificity estimates of the three modalities for each reader as well as for all the readers combined are listed in Table 2. The sensitivity of the combined modality was significantly higher than that of FFDM alone for four readers (p=0.004 for reader A; p<0.001 for reader B; p<0.001 for reader D; and p=0.035 for reader F). For readers A and B, the sensitivity of DBT alone was also higher than that of FFDM alone (p=0.024 for reader A; p=0.015 for reader B). For reader D, the sensitivity of the combined modality was higher than that of DBT alone (p=0.035). Regarding specificity, two readers showed significantly higher specificity for the combined modality than for FFDM alone (p<0.001 for reader E; p=0.008 for reader F). The specificity of the combined modality was also higher than that of DBT alone for two readers (p=0.022 for reader E; p=0.009 for reader F). The overall sensitivity of the combined modality was significantly higher than that of FFDM alone (80.0% vs. 73.2%, p<0.001); and the overall sensitivity of DBT alone was also higher than that of FFDM alone (78.3% vs. 73.2%, p=0.007). However, differences in overall specificities between the three modalities were not statistically significant.
Among the 129 cancers (113 invasive cancers and 16 DCIS), 48 cancers (37 invasive cancers and 11 DCIS) that were missed on FFDM alone were detected using the combined modality by at least one reader (29 cancers by one reader, 13 cancers by two readers, 2 cancers by three readers, 2 cancers by two readers, and 2 cancers by six readers) (Figure 1).
Distribution of the lesions' characteristics (clinical, mammographic, and histopathologic characteristics) according to the number of readers who detected the lesions with combined DBT and FFDM are summarized in Table 3. Of 129 cancers, 98 lesions (76.0%) were detected by five or more readers, 9 lesions (7.0%) by three or four readers, and 12 lesions (9.0%) by one or two readers. Ten lesions (8.0%) were not detected by any reader. Ordinal logistic analysis revealed mammographic lesion size (p=0.009), patient's symptoms (p=0.003), mammographic identification of microcalcifications (p<0.001), architectural distortion (p=0.049), and the presence of masses (p<0.001) to be significantly associated with cancer detectability using the combined modality. Patient's age, distance from the nipple to the lesion, mammographic density, lesion location quadrant, and pathology were not significantly associated with detectability of the lesions. In multivariate analysis, mammographic findings of masses (odds ratio, 76.0; p<0.001) or microcalcifications (odds ratio, 61.8; p<0.001) were significantly associated with a higher cancer detection rate.
Features of the 10 breast cancers that were not detected on the combined modality by any reader are shown in Table 4. Among these lesions, there were two DCIS and eight IDCs. Of the 10 lesions, four showed focal asymmetry and one showed a subtle architectural distortion on an unblinded review by two dedicated radiologists, although none of the six blinded readers detected the lesions on prospective review. The lesions were all misinterpreted as focal asymmetric prominent parenchyma, even in the case with a fatty breast background (patient number 6). Five cases showed no abnormal findings even on retrospective unblinded review of combined DBT and FFDM. All five cancers that were occult on combined DBT and FFDM were obscured by dense parenchymal tissue, and BI-RADS breast density was interpreted as heterogeneously dense or extremely dense in each case (Figure 2).
In our study, the overall diagnostic performance and sensitivity of combined DBT and FFDM in the diagnostic setting were observed to be superior to FFDM alone. Nonetheless, even with combined DBT and FFDM, cancers not presenting as masses and cancers without calcifications were still difficult to detect. In addition, we found that DBT alone offered superior diagnostic performance and showed significantly higher sensitivity than FFDM alone. Compared with FFDM alone, the combined modality was able to detect 48 additional cancers out of a total of 129 cancers.
Prior investigators have reported on the interpretive advantages of adding DBT to conventional FFDM in the diagnostic and screening setting and demonstrated a reduction in recall rate and gain in diagnostic accuracy [1213151620]. Indeed, a recent review study suggested that integration of DBT with conventional FFDM might substantially improve breast cancer detection rates [21]. Similar to previous studies, we found that combined DBT and FFDM offers superior diagnostic accuracy and sensitivity to FFDM alone even though our study was only performed in a diagnostic setting. Our results differed from those of previous studies in that the overall specificity was not significantly improved with the addition of DBT. This might be because we only included women who had clinical signs and symptoms of breast lesions, or an abnormality detected at a screening examination. Even though DBT alone performed similarly to the combined modality in our study, current evidence favors using DBT as an adjunct to 2D mammography rather than as a stand-alone modality [22]. Two-dimensional images are essential for comparison with prior mammograms to evaluate developing asymmetry. Developing asymmetry is defined as focal asymmetry that is new, larger, or denser on the current examination than it was on a previous mammogram [23]. It is an uncommon mammographic finding, but represents malignancy in 26.7% of diagnostic imaging-detected cases [24]. In addition, some studies report that 2D mammography is better than 3D DBT alone in the evaluation of calcifications [81225]. More research is required in this area.
It is well documented that breast cancer can be obscured by surrounding fibroglandular tissue as the two have similar densities on conventional mammography including FFDM, particularly in women with dense breasts [4]. In this regard, DBT has been shown to improve lesion conspicuity by reducing tissue overlap, providing comparable results to mammographic spot view in the characterization of mass margins [1326]. Yet, despite the addition of DBT to FFDM in our study, 10 cancers (8.0%) were not detected by any reader. Of the 10 cancers missed in our study, four showed focal asymmetry, one showed subtle architectural distortion, and five were occult on both DBT and FFDM even on unblinded review. The five occult cancers were located in fibroglandular tissue and did not show an enhanced margin on DBT due to superimposed breast tissue. It is evident that cancers surrounded by fibroglandular tissue that do not have a margin highlighted by fat, do not produce calcifications, and do not show architectural distortions are less conspicuous on mammography [27]. These cancers could then be occult on DBT for the same reasons. Hooley et al. [28] reported that the radiologists in their study were not able to identify two IDCs on DBT, even with retrospective review, which had been initially detected by whole breast US. Other studies reported that US detected more cancers than DBT in women with mammography negative dense breasts [2930]. It is important to note that some cancers were occult on both DBT and FFDM because of surrounding fibroglandular tissue, rather than because of overlapping tissue.
Our study had several limitations. First, we included only diagnostic women in our study; therefore our results cannot be generalized to include the screening setting. Second, although we recruited our patients prospectively, the image review was done retrospectively and prior imaging was not available for comparison. As a result of these factors, our results may have been different from those obtained in the clinical setting. Thus, further studies including a larger number of normal and benign cases as would be seen in the clinical setting are warranted to confirm the performance of this technique. Third, even though our radiologists were specialized in breast imaging and received additional training in the interpretation of DBT images, they had relatively little experience with this modality as compared to FFDM, and this may have affected the results of our study.
In conclusion, DBT combined with FFDM yielded a better diagnostic performance and sensitivity than FFDM alone in the diagnostic setting. DBT alone was also shown to provide a superior diagnostic performance and sensitivity to FFDM alone. Nonetheless, even using the combination of DBT and FFDM, multivariate analysis revealed that cancers not presenting as masses and cancers without calcifications may still be difficult to detect.
Figures and Tables
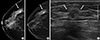 | Figure 1Invasive ductal carcinoma in a 50-year-old woman. (A) Craniocaudal view of full-field digital mammography (FFDM) demonstrates a mass largely obscured by overlying breast tissue (arrow) which was misinterpreted as being negative by four of the six blinded readers. (B) Craniocaudal view of digital breast tomosynthesis (DBT), however, clearly demonstrates the mass (arrow) and all six readers detected the mass on combined FFDM and DBT. (C) Ultrasonography image shows a 1.5-cm irregular, hypoechoic mass with indistinct margins (arrows). |
 | Figure 2Invasive ductal carcinoma in a 47-year-old woman. (A) Craniocaudal view of full-field digital mammography shows a heterogeneously dense breast tissue which was interpreted as negative by six blinded readers. (B) Craniocaudal view of digital breast tomosynthesis also shows heterogeneously dense breast tissue at the same location. (C) Ultrasonography image shows a 1.1-cm irregular, hypoechoic mass with indistinct margins (arrows). |
Table 1
Figures of merit for FFDM alone, DBT alone, and combined FFDM and DBT for each reader, as well as overall figures of merit for all readers

Table 2
Sensitivities and specificities of FFDM alone, DBT alone, and the combined modality for each reader, as well as overall sensitivities and specificities for all readers

FFDM=full-field digital mammography; DBT=digital breast tomosynthesis.
*p-values indicate comparison between FFDM alone and DBT alone; †p-values indicwate comparison between DBT alone and combined modality; ‡p-values indicate comparison between FFDM alone and combined modality; §Numbers are percentages, with raw data in parentheses.
Table 3
Univariate analysis of the association of lesion characteristics and the number of readers who detected lesions with combined FFDM and DBT in 129 cancers

Table 4
Features of 10 breast cancers not detected with combined FFDM and DBT by any reader

References
1. Youlden DR, Cramb SM, Dunn NA, Muller JM, Pyke CM, Baade PD. The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol. 2012; 36:237–248.

2. Paap E, Holland R, den Heeten GJ, van Schoor G, Botterweck AA, Verbeek AL, et al. A remarkable reduction of breast cancer deaths in screened versus unscreened women: a case-referent study. Cancer Causes Control. 2010; 21:1569–1573.

3. Tabár L, Vitak B, Chen TH, Yen AM, Cohen A, Tot T, et al. Swedish two-county trial: impact of mammographic screening on breast cancer mortality during 3 decades. Radiology. 2011; 260:658–663.

4. Pisano ED, Gatsonis C, Hendrick E, Yaffe M, Baum JK, Acharyya S, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005; 353:1773–1783.

5. Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007; 356:227–236.

6. Carney PA, Miglioretti DL, Yankaskas BC, Kerlikowske K, Rosenberg R, Rutter CM, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003; 138:168–175.

7. Gur D, Abrams GS, Chough DM, Ganott MA, Hakim CM, Perrin RL, et al. Digital breast tomosynthesis: observer performance study. AJR Am J Roentgenol. 2009; 193:586–591.

8. Poplack SP, Tosteson TD, Kogel CA, Nagy HM. Digital breast tomosynthesis: initial experience in 98 women with abnormal digital screening mammography. AJR Am J Roentgenol. 2007; 189:616–623.

9. Good WF, Abrams GS, Catullo VJ, Chough DM, Ganott MA, Hakim CM, et al. Digital breast tomosynthesis: a pilot observer study. AJR Am J Roentgenol. 2008; 190:865–869.

10. Andersson I, Ikeda DM, Zackrisson S, Ruschin M, Svahn T, Timberg P, et al. Breast tomosynthesis and digital mammography: a comparison of breast cancer visibility and BIRADS classification in a population of cancers with subtle mammographic findings. Eur Radiol. 2008; 18:2817–2825.

11. Teertstra HJ, Loo CE, van den Bosch MA, van Tinteren H, Rutgers EJ, Muller SH, et al. Breast tomosynthesis in clinical practice: initial results. Eur Radiol. 2010; 20:16–24.

12. Rafferty EA, Park JM, Philpotts LE, Poplack SP, Sumkin JH, Halpern EF, et al. Assessing radiologist performance using combined digital mammography and breast tomosynthesis compared with digital mammography alone: results of a multicenter, multireader trial. Radiology. 2013; 266:104–113.

13. Skaane P, Gullien R, Bjørndal H, Eben EB, Ekseth U, Haakenaasen U, et al. Digital breast tomosynthesis (DBT): initial experience in a clinical setting. Acta Radiol. 2012; 53:524–529.

14. Bernardi D, Ciatto S, Pellegrini M, Tuttobene P, Fanto' C, Valentini M, et al. Prospective study of breast tomosynthesis as a triage to assessment in screening. Breast Cancer Res Treat. 2012; 133:267–271.

15. Skaane P, Bandos AI, Gullien R, Eben EB, Ekseth U, Haakenaasen U, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013; 267:47–56.

16. Haas BM, Kalra V, Geisel J, Raghu M, Durand M, Philpotts LE. Comparison of tomosynthesis plus digital mammography and digital mammography alone for breast cancer screening. Radiology. 2013; 269:694–700.

17. Friedewald SM, Rafferty EA, Rose SL, Durand MA, Plecha DM, Greenberg JS, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014; 311:2499–2507.

18. McCarthy AM, Kontos D, Synnestvedt M, Tan KS, Heitjan DF, Schnall M, et al. Screening outcomes following implementation of digital breast tomosynthesis in a general-population screening program. J Natl Cancer Inst. 2014; 106:dju316.

19. Gur D, Bandos AI, Rockette HE, Zuley ML, Sumkin JH, Chough DM, et al. Localized detection and classification of abnormalities on FFDM and tomosynthesis examinations rated under an FROC paradigm. AJR Am J Roentgenol. 2011; 196:737–741.

20. Ciatto S, Houssami N, Bernardi D, Caumo F, Pellegrini M, Brunelli S, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol. 2013; 14:583–589.

21. Houssami N, Skaane P. Overview of the evidence on digital breast tomosynthesis in breast cancer detection. Breast. 2013; 22:101–108.

22. Chesebro AL, Winkler NS, Birdwell RL, Giess CS. Developing asymmetry at mammography: correlation with US and MR imaging and histopathologic findings. Radiology. 2016; 279:385–394.

23. D'Orsi CJ, Sickles EA, Mendelson EB, Morris EA. ACR BI-RADS® Atlas: Breast Imaging and Reporting and Data System. 5th ed. Reston: American College of Radiology;2013.
24. Leung JW, Sickles EA. Developing asymmetry identified on mammography: correlation with imaging outcome and pathologic findings. AJR Am J Roentgenol. 2007; 188:667–675.

25. Spangler ML, Zuley ML, Sumkin JH, Abrams G, Ganott MA, Hakim C, et al. Detection and classification of calcifications on digital breast tomosynthesis and 2D digital mammography: a comparison. AJR Am J Roentgenol. 2011; 196:320–324.

26. Noroozian M, Hadjiiski L, Rahnama-Moghadam S, Klein KA, Jeffries DO, Pinsky RW, et al. Digital breast tomosynthesis is comparable to mammographic spot views for mass characterization. Radiology. 2012; 262:61–68.

28. Hooley RJ, Geisel JL, Raghu M, Durand MA, Gross CP, Busch SH, et al. Performance of whole breast ultrasound in women with dense breasts following 3D tomosynthesis mammography. In : The 99th Radiological Society of North America Scientific Assembly and Annual Meeting; 2013. Abstract #SSQ01-06.
29. Mariscotti G, Houssami N, Durando M, Bergamasco L, Campanino PP, Ruggieri C, et al. Accuracy of mammography, digital breast tomosynthesis, ultrasound and MR imaging in preoperative assessment of breast cancer. Anticancer Res. 2014; 34:1219–1225.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download