Abstract
Purpose
As the numbers of cancer cases and survivors increase, the incidence and natural history of chemotherapy-induced cardiotoxicities in patients with breast cancer may also be expected to change. The present study aimed to investigate the incidence and predictors of chemotherapy-induced left ventricular dysfunction (LVD) in patients with breast cancer.
Methods
From 2003 to 2010, 712 female patients with breast cancer (55.7±10.7 years) were enrolled and divided into the LVD group (n=82, 56.7±10.1 years) and the non-LVD group (n=630, 55.6±10.8 years). Baseline clinical and treatment-related variables were compared.
Results
Chemotherapy-induced LVD developed in 82 cases (11.4%). Low body mass index (BMI), low triglyceride level, advanced cancer stage, and the use of doxorubicin, paclitaxel, trastuzumab, or radiotherapy were significant predictors of LVD in a univariate analysis. In a multivariate analysis, low BMI, advanced cancer stage, and the use of target therapy with trastuzumab were independent predictors of chemotherapy-induced LVD. Chemotherapy-induced LVD was recovered in 53 patients (64.6%), but left ventricular function was not recovered in 29 patients (35.4%).
Conclusion
Chemotherapy-induced LVD was not uncommon and did not reduce in many of our patients with breast cancer. Low BMI, advanced cancer stage, and the use of trastuzumab were independent predictors of chemotherapy-induced LVD in patients with breast cancer. The development of chemotherapy-induced LVD should be carefully monitored in patients with breast cancer who are receiving trastuzumab therapy, have poor nutritional status, and advanced cancer stage.
With the technical advances in the early detection of and therapeutic strategies for cancer, cancer survival has been significantly improved and cancer recurrence has been significantly reduced in recent years. Despite this improvement in cancer therapy, however, several treatment-related adverse effects have become important issues for cancer survivors [1].
Chemotherapy may exert adverse effects on various organ systems, such as bone marrow suppression, neurotoxicity, gastrointestinal problems, hepatotoxicity, nephrotoxicity, and cardiotoxicity. Cardiotoxicity, including heart failure with left ventricular dysfunction (LVD), is one of the most serious adverse effects of chemotherapy and becomes an important morbidity and mortality in cancer survivors [23]. Cardiotoxicity depends on various factors, including the types of chemotherapeutic regimens, combined use of other drugs, radiotherapy (RT), or comorbidities of the patients.
Breast cancer is the second most common malignancy and leading cause of cancer-related death in Korean women [4]. Adjuvant or neoadjuvant chemotherapy using anthracycline- or taxane-based regimens or trastuzumab reduced breast cancer mortality by up to 25%–30% [5]. Despite the beneficial effects of anthracyclines on breast cancer, for several decades, the use of anthracyclines has been limited by the development of chemotherapy-induced cardiotoxicity that results in LVD and congestive heart failure [6]. Initial evidence indicated that LVD was closely related to cumulative doses of anthracyclines, and the repeated administration of these agents can result in permanent cellular and interstitial damage, and is frequently associated with refractory heart failure [7]. To reduce cardiotoxicity, non-anthracycline-containing new therapeutic agents have been developed, but these new agents may also result in cardiotoxicity. Cardiotoxicity by non-anthracycline-containing new agents is usually more transient, reversible, and unrelated to the cumulative dose than anthracycline-induced cardiotoxocity, and thus associated with better prognosis [8910]. The life expectancy of patients with breast cancer has increased owing to these new agents, but cardiotoxicity associated with non-anthracycline-containing new agents has also become an important issue in breast cancer survivors.
Although breast cancer is an important morbidity and mortality in Korean women, the incidence, predictors, and natural history of cardiotoxicity have been poorly studied. Therefore, the aim of this study was to investigate the incidence, predictors, and clinical outcomes of chemotherapy-induced LVD in Korean patients with breast cancer.
The present study was a single-center retrospective observational study, and the study protocol was approved by Chonnam National University Hospital Institutional Review Board (number: 2015-05-092). From January 2003 to December 2010, 5,050 female patients with breast cancer were treated with surgery and chemotherapy. After exclusion of 4,338 patients, 712 patients who underwent baseline echocardiography before chemotherapy and serial follow-up echocardiography after chemotherapy were finally enrolled. The reasons for the exclusion were as follows: (1) no baseline or follow-up echocardiography (n=4,234); (2) LVD defined as a left ventricular (LV) ejection fraction (EF) of <55% on baseline echocardiography (n=36); (3) significant valvular heart disease defined as valvular stenosis or regurgitation of more than moderate degree on baseline echocardiography (n=15); (4) known coronary artery disease (n=45); and (5) hypertrophic or restrictive cardiomyopathy (n=8).
According to LVEF on follow-up echocardiography after chemotherapy, the patients were divided into two groups as follows: LVD group (group I, n=82, 56.7±10.1 years) and non-LVD group (group II, n=630, 55.6±10.8 years).
All the patients received neoadjuvant or adjuvant chemotherapy that consisted of four to six cycles of anthracycline-based, taxane-based, or combined regimens [1112]. Modified radical mastectomy or breast conservative surgery was performed in patients who had no evidence of disease progression. RT using conventional techniques after considering the clinical tumor size and/or staging was performed for patients treated with conservative breast surgery. Trastuzumab was administered for 1 year in patients with human epidermal growth factor receptor 2 (HER2) overexpression at the discretion of the physician in charge [1314].
Echocardiographic images from various echocardiographic windows were obtained by using a digital ultrasonographic equipment system (Vivid 7; GE Vingmed Ultrasound, Horten, Norway). Digital cine loops were obtained for subsequent offline analysis. All of the data were analyzed by using the computerized offline software package (EchoPAC PC 6.0.0; GE Vingmed Ultrasound). Baseline echocardiography was performed before starting chemotherapy for breast cancer, and follow-up echocardiography was performed just after finishing all scheduled cycles of the chemotherapy regimen. In cases of symptoms or signs of heart failure, however, follow-up echocardiography was performed as soon as possible, even though all the scheduled cycles of chemotherapy were not completed.
Routine echocardiographic examinations were performed in accordance with the recommendation of the current guideline [15]. LV volume and EF were measured by using Simpson's biplane method, and left atrial (LA) volume was measured by using the biplane area-length method. LV and LA volume indexes were calculated by using the LV and LA volumes divided by the body surface area. The intraobserver and interobserver variabilities of Simpson's method were 4%±5% and 5%±4% (absolute difference divided by the mean measurement value). The early (E) and late diastolic velocities (A) of the mitral inflow were measured by using pulsed wave Doppler from the apical 4-chamber view, with the sample volume positioned at the tip of the mitral leaflets. The early (e′), late diastolic (a′), and systolic velocities of the mitral septal annulus were measured by using tissue Doppler imaging in the apical four chamber view. Diastolic function was classified as “normal,” “relaxation abnormality,” “pseudonormal,” and “restrictive physiology,” according to the current guideline [16].
Regardless of symptoms or signs of heart failure, chemotherapy-induced cardiotoxicity was defined as the development of de novo LVD on follow-up echocardiography after chemotherapy. In the present study, LVD was defined according to the current guideline (Cardiac Review and Evaluation Committee of Trastuzumab-associated Cardiotoxicity) as a LVEF of <55% or the decrease in LVEF of >10% from the baseline LVEF on follow-up echocardiography after chemotherapy [17].
The SPSS version 18.0 for Windows (SPSS Inc., Chicago, USA) was used for the statistical analysis. Data are presented as percentages or mean±standard deviation. The differences in the categorical variables were evaluated by using the chi-square test, and the continuous variables were compared by using the independent t-test. Event-free survival rate was evaluated by using the Kaplan-Meier analysis, and event rates were compared by using the log-rank test. To identify the independent predictor of chemotherapy-induced LVD, multivariate logistic regression analysis was applied to the significant variables in the univariate analysis. A p-value of <0.05 was considered as statistically significant.
The baseline characteristics of the groups are summarized in Table 1. Chemotherapy-induced LVD developed in 82 cases (11.5%). Body mass index (BMI) and triglyceride level were significantly lower, whereas advanced-stage cancer was more frequent in the LVD group than in the non-LVD group. Estrogen or progesterone receptor was more frequently positive in the non-LVD group, whereas HER2 was more frequently positive in the LVD group. A detailed baseline tumor staging is shown in Figure 1. Other baseline characteristics did not significantly differ between the groups.
The baseline and follow-up echocardiographic findings are summarized in Table 2. On baseline echocardiography, LV end diastolic dimensions were significantly larger and EF was significantly lower in the LVD group than in the non-LVD group. Other echocardiographic findings, including diastolic function grade, did not significantly differ between the groups.
Follow-up echocardiography was performed just after finishing all the scheduled cycles of chemotherapy regimens in most of the study patients, but early follow-up echocardiography was performed in 14 patients because of symptoms suggesting heart failure. On follow-up echocardiography, LV end systolic dimension was significantly larger and EF was significantly lower in the LVD group than in the non-LVD group. Other echocardiographic findings did not significantly differ between the groups.
LVEF was significantly decreased not only in the LVD group (64.2%±6.3% at baseline vs. 53.9%±6.1% at follow-up, p<0.001) but also in the non-LVD group on follow-up echocardiography (68.4%±5.2% at baseline vs. 64.1%±5.3% at follow-up, p<0.001). Advanced diastolic dysfunction of >grade 2 was also significantly increased not only in the LVD group (2.4% vs. 11.2%, p<0.001) but also in the non-LVD group (1.4% vs. 12.7%, p=0.003) on follow-up echocardiography.
Nonsurgical treatment-related findings are summarized in Table 3. The LVD group received more chemotherapy cycles than the non-LVD group. Doxorubicin, paclitaxel, and trastuzumab were more frequently used in the LVD group than in the non-LVD group. Other chemotherapy-related findings, including the dosage used for chemotherapeutic agents, did not significantly differ between the groups. RT was more frequently applied in the non-LVD group than in the LVD group, but the frequency of the RT cycle and radiation dose did not significantly differ between the groups.
To identify the independent predictors of chemotherapy-related LVD, multivariate regression analysis, including significant variables in the univariate analysis, was performed. Low BMI, advanced stage, and target therapy with trastuzumab correlated with LVD (r=0.109, p=0.004; r=0.194, p<0.001; and r=0.242, p<0.001) and were significant independent predictors of chemotherapy-induced LVD (Table 4). Cumulative LVD-free survival in the Kaplan-Meier analysis was significantly lower in the patients with lower BMI or concomitant target therapy with trastuzumab (Figure 2).
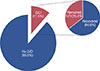
Symptomatic heart failure developed in 59 of 82 patients with LVD (71.2%), and the patients were consulted and managed at the Heart Failure Clinic. Chemotherapy was discontinued in 14 of the 82 patients with LVD (17.1%). To monitor LV function, additional follow-up echocardiography was performed at 12 months after the index event of LVD. LV function was recovered in 53 cases (64.6%), whereas LVD was not recovered in the remaining 29 cases (35.4%) (Figure 3). LVD was improved in nine of the 14 patients who discontinued chemotherapy and in 44 of 68 patients who completed the scheduled chemotherapy. Chemotherapy cessation was not associated with the recovery of LV function.
During 54 months of clinical follow-up, 78 patients died (18 deaths in the LVD group vs. 60 deaths in the non-LVD). Mortality rate was significantly higher in the LVD group than in the non-LVD group (21.9% vs. 9.5%, p=0.002), but mortality rate did not significantly differ according to LV function recovery (24.1% in the nonrecovered group vs. 20.7% in the recovered group, p=0.464).
The present study evaluated chemotherapy-induced LVD in Korean patients with breast cancer and demonstrated several important findings. First, chemotherapy-induced LVD was not uncommon in the patients with breast cancer. Second, low BMI, advanced cancer stage, and the use of trastuzumab as target therapy were independent predictors of chemotherapy-induced LVD in the patients with breast cancer. Third, about one third of the patients with chemotherapy-induced LVD did not show LV function recovery, even though LV function was recovered in two thirds of the patients with chemotherapy-induced LVD. Fourth, the development of chemotherapy-induced LVD was associated with poor prognosis, even though the LV function recovery in the LVD group was not associated with mortality.
As breast cancer survival has increased owing to diagnosis and treatment in the early stage, the side effects of anticancer therapies, such as cardiotoxicities, have become an important issue. For several decades, chemotherapy-induced cardiomyopathy was almost exclusively associated with the use of anthracyclines, which causes acute and chronic damage at the cellular level [18]. However, with the introduction of new therapeutic agents such as the monoclonal antibody trastuzumab, the incidence or pathogenesis of cardiotoxicity has changed [19]. In the study of Hershman and Shao [20] that included 2,992 patients with breast cancer who received anthracycline-based chemotherapy, LVD developed in 8.5% of the patients. In adjuvant trials that incorporated trastuzumab with doxorubicin plus cyclophosphamide and paclitaxel, approximately 5% to 7% of patients with breast cancer experienced cardiotoxicity. In a trial of monotherapy with trastuzumab, cardiotoxicity was noted in 2% to 4.7% of patients with metastatic breast cancer [21]. In a retrospective analysis, the incidence of cardiac dysfunction was 2.6% in patients who received first-line, single-agent trastuzumab and 8.5% in patients who received trastuzumab as second- or third-line therapy [22]. In the present study, anthracycline-based regimen with or without trastuzumab was used as a chemotherapy regimen for breast cancer, and chemotherapy-induced LVD developed in 82 of the 712 patients (11.5%). As the present study analyzed only 712 patients with both baseline and follow-up echocardiographies among 5,050 breast cancer patients, its results cannot reflect the true incidence of cardiotoxicity in the whole patient population with breast cancer. Further prospective studies that included all of the breast cancer population are needed to evaluate the true incidence of cardiotoxicity in Korea.
The present study showed that LVEF and diastolic grade deteriorated not only in the LVD group but also in the non-LVD group on follow-up echocardiography. This may suggest that subclinical LVD was more frequent than that under definition. Therefore, the early identification of high-risk patients for chemotherapy-induced LVD would be clinically important. Older age, hypertension, preexisting heart disease, mediastinal irradiation, treatment with cyclophosphamide, paclitaxel, or trastuzumab are well-known factors associated with the increased risk of anthracycline-induced cardiotoxicity [23]. Age, the prevalence of hypertension, and the use or cumulative dose of cyclophosphamide, paclitaxel, or trastuzumab did not significantly differ between the patients with and those without LVD in the present study. Meanwhile, low BMI, advanced cancer stage, and the use of trastuzumab as a target therapy were independent predictors of chemotherapy-induced LVD in the Korean patients with breast cancer. Therefore, patients with these characteristics may be considered as a high-risk group and the development of chemotherapy-induced LVD should be closely monitored. One of the most important characteristic in the pathogenesis of doxorubicin cardiotoxicity is that the risk of doxorubicin cardiotoxicity is dose dependent and increases dramatically when the cumulative doses exceed 500 mg/m2. In a previous study that included Korean patients with breast cancer, the cumulative dose of doxorubicin of ≥300 mg/m2 was also associated with the development of subclinical cardiotoxicity [24]. However, the cumulative dose of anthracycline did not significantly differ between the patients with and those without LVD in the present study.
Low BMI was an independent risk factor of chemotherapy-induced LVD in the present study, even though it was not a well-known risk factor of chemotherapy-induced cardiotoxicity. The associated mechanism between low BMI and chemotherapy-induced LVD is unclear. In contrast to the result of the present study, the previous study suggested that elevated body weight is a risk factor for anthracycline induced cardiotoxicity [2526]. This discrepant result regarding the association between obesity and chemotherapy-induced LVD may come from a selection bias because many patients with breast cancer were excluded in the present study. The other possible assumption is that low BMI may be a reflection of poor nutritional status, and patients with poor nutritional status is more prone to the development of heart failure [27]. To clarify the association between obesity and chemotherapy-induced LVD, larger prospective studies that include the whole patient population with breast cancer are needed.
Although the reversibility of chemotherapy-induced LVD has been described, the natural history of chemotherapy-induced LVD is still not well documented in terms of the reversibility of LV function and cardiovascular outcomes. LV function was recovered in two thirds of the patients with chemotherapy-induced LVD in the present study, but chemotherapy-induced LVD persisted in about one third of the patients. Anthracycline-induced cardiotoxicity, described as type 1 cardiotoxicity, is associated with cardiomyocyte death resulting in irreversible injury. By contrast, trastuzumab-induced cardiotoxicity, described as type 2 cardiotoxicity, is associated with dysfunction of cardiac muscle cell, rather than cell death, and is usually reversible by the discontinuation of chemotherapeutic agents and medical therapy for heart failure [28]. Non-recovery of LV function in the present study may be associated with the combination of type 1 and 2 cardiotoxicities in the setting of risk factors.
The present study has several potential limitations. First, the present study has all limitations of a retrospective analysis. As discussed already, the present study only analyzed 712 patients with both baseline and follow-up echocardiographies among 5,050 breast cancer patients; thus, selection bias would be a major limitation. The unusually high HER2 positivity in both the LAD and non-LVD groups may also come from the selection bias in the present study. Second, the present study included patients with both early- and advanced-stage breast cancer. Therefore, patients with advanced breast cancer may have more cycles or doses of chemotherapy or more chances of using doxorubicin, paclitaxel, or trastuzumab than patients with early breast cancer. These factors would affect the incidence or natural history of chemotherapy-induced LVD, and the mortality rate can be affected by these factors. Third, because the timing of the follow-up echocardiography was not the same among the patients, the onset of chemotherapy-induced LVD could not be estimated in the present study. Fourth, the baseline LVEF was lower in the LVD group than in the non-LVD group, even though LVEF was within the normal limit in all the patients. This factor should be considered as one of the possible causes of LVD. Fifth, the prescribed medications for heart failure after the diagnosis of chemotherapy-induced LVD were not analyzed in the present study. Therefore, the data on LV function recovery may be affected by these medications. Sixth, coronary angiography was not routinely performed in the LVD group; thus, the possibility of LVD due to coronary artery disease cannot be excluded. As the patients with known coronary artery disease were already excluded in the study and most of the patients with LVD showed global hypokinesia, the risk of coronary artery disease would be minimized.
In conclusion, despite these potential limitations, the results of the present study demonstrated that chemotherapy-induced LVD was not uncommon, and low BMI, advanced cancer stage, and trastuzumab use were independent predictors in the patients with breast cancer. Therefore, the development of chemotherapy-induced LVD should be carefully monitored in patients with breast cancer who are receiving trastuzumab therapy, have poor nutritional status, and advanced cancer stage.
Figures and Tables
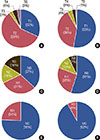 | Figure 1Baseline TNM staging of breast cancer. The percent of advanced T stage were significant higher in left ventricular dysfunction (LVD) group (A) than in no LVD group (B). The percent of advanced N stage were significant higher in LVD group (C) than in no LVD group (D). The percent of advanced M stage were significant higher in LVD group (E) than in no LVD group (F). |
 | Figure 2Left ventricular dysfunction (LVD)-free survival according to the body mass index (BMI) (A) and use of trastuzumab (B) on Kaplan-Meier curve analysis. (A) LVD-free survival rate was significant higher in patients with higher BMI than those with lower BMI. (B) LVD-free survival rate was significant higher in patients without expose to trastuzumab than those treated with trastuzumab. |
Table 1
Baseline characteristics between the groups
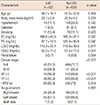
Table 2
Baseline and follow-up echocardiographic findings between the groups

Data are presented as mean±SD or number (%).
LVD=left ventricular dysfunction; LVEDD=left ventricular end-diastolic dimension; LVESD=left ventricular end-systolic dimension; EF=ejection fraction; LAD=left atrial dimension; LAVI=left atrial volume index; E=early diastolic velocity of mitral inflow; DT=deceleration time of mitral inflow; e′=early diastolic velocity of mitral septal annulus; s′=systolic velocity of mitral septal annulus; RVSP=right ventricular systolic pressure.
Table 3
Nonsurgical treatment related characteristics between the groups

Table 4
Independent predictors of chemotherapy induced left ventricular dysfunction

Notes
References
1. Minami M, Matsumoto S, Horiuchi H. Cardiovascular side-effects of modern cancer therapy. Circ J. 2010; 74:1779–1786.

2. Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009; 53:2231–2247.
3. Raschi E, Vasina V, Ursino MG, Boriani G, Martoni A, De Ponti F. Anticancer drugs and cardiotoxicity: insights and perspectives in the era of targeted therapy. Pharmacol Ther. 2010; 125:196–218.

4. Lee JH, Yim SH, Won YJ, Jung KW, Son BH, Lee HD, et al. Population-based breast cancer statistics in Korea during 1993-2002: incidence, mortality, and survival. J Korean Med Sci. 2007; 22:Suppl. S11–S16.

5. Kaufmann M, Hortobagyi GN, Goldhirsch A, Scholl S, Makris A, Valagussa P, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: an update. J Clin Oncol. 2006; 24:1940–1949.

6. Ryberg M, Nielsen D, Skovsgaard T, Hansen J, Jensen BV, Dombernowsky P. Epirubicin cardiotoxicity: an analysis of 469 patients with metastatic breast cancer. J Clin Oncol. 1998; 16:3502–3508.

7. Sawyer DB, Peng X, Chen B, Pentassuglia L, Lim CC. Mechanisms of anthracycline cardiac injury: can we identify strategies for cardioprotection? Prog Cardiovasc Dis. 2010; 53:105–113.

8. Telli ML, Hunt SA, Carlson RW, Guardino AE. Trastuzumab-related cardiotoxicity: calling into question the concept of reversibility. J Clin Oncol. 2007; 25:3525–3533.

9. Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002; 20:1215–1221.

10. Yoon GJ, Telli ML, Kao DP, Matsuda KY, Carlson RW, Witteles RM. Left ventricular dysfunction in patients receiving cardiotoxic cancer therapies are clinicians responding optimally? J Am Coll Cardiol. 2010; 56:1644–1650.

11. Bear HD. Primary chemotherapy for operable breast cancer: the NSABP experience. Breast Cancer Res. 2005; 7:Suppl 1. S17.

12. Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003; 21:4165–4174.

13. Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011; 365:1273–1283.

14. Joensuu H, Bono P, Kataja V, Alanko T, Kokko R, Asola R, et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer Trial. J Clin Oncol. 2009; 27:5685–5692.

15. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015; 28:1–39.e14.

16. Appleton CP, Galloway JM, Gonzalez MS, Gaballa M, Basnight MA. Estimation of left ventricular filling pressures using two-dimensional and Doppler echocardiography in adult patients with cardiac disease: additional value of analyzing left atrial size, left atrial ejection fraction and the difference in duration of pulmonary venous and mitral flow velocity at atrial contraction. J Am Coll Cardiol. 1993; 22:1972–1982.

17. Martín M, Esteva FJ, Alba E, Khandheria B, Pérez-Isla L, García-Sáenz JA, et al. Minimizing cardiotoxicity while optimizing treatment efficacy with trastuzumab: review and expert recommendations. Oncologist. 2009; 14:1–11.

18. Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003; 97:2869–2879.

19. Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, et al. 2-Year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007; 369:29–36.

20. Hershman DL, Shao T. Anthracycline cardiotoxicity after breast cancer treatment. Oncology (Williston Park). 2009; 23:227–234.
21. Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002; 20:719–726.

22. Perez EA, Suman VJ, Davidson NE, Sledge GW, Kaufman PA, Hudis CA, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol. 2008; 26:1231–1238.

23. Volkova M, Russell R 3rd. Anthracycline cardiotoxicity: prevalence, pathogenesis and treatment. Curr Cardiol Rev. 2011; 7:214–220.

24. Chung WB, Yi JE, Jin JY, Choi YS, Park CS, Park WC, et al. Early cardiac function monitoring for detection of subclinical Doxorubicin cardiotoxicity in young adult patients with breast cancer. J Breast Cancer. 2013; 16:178–183.

25. Dranitsaris G, Rayson D, Vincent M, Chang J, Gelmon K, Sandor D, et al. The development of a predictive model to estimate cardiotoxic risk for patients with metastatic breast cancer receiving anthracyclines. Breast Cancer Res Treat. 2008; 107:443–450.

26. Fumoleau P, Roché H, Kerbrat P, Bonneterre J, Romestaing P, Fargeot P, et al. Long-term cardiac toxicity after adjuvant epirubicin-based chemotherapy in early breast cancer: French Adjuvant Study Group results. Ann Oncol. 2006; 17:85–92.

27. Voulgari C, Tentolouris N, Dilaveris P, Tousoulis D, Katsilambros N, Stefanadis C. Increased heart failure risk in normal-weight people with metabolic syndrome compared with metabolically healthy obese individuals. J Am Coll Cardiol. 2011; 58:1343–1350.

28. Groarke JD, Nohria A. Anthracycline cardiotoxicity: a new paradigm for an old classic. Circulation. 2015; 131:1946–1949.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download