1. Gralow JR, Burstein HJ, Wood W, Hortobagyi GN, Gianni L, von Minckwitz G, et al. Preoperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol. 2008; 26:814–819. PMID:
18258991.

2. Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005; 97:188–194. PMID:
15687361.

3. Chia S, Swain SM, Byrd DR, Mankoff DA. Locally advanced and inflammatory breast cancer. J Clin Oncol. 2008; 26:786–790. PMID:
18258987.

4. Buchholz TA, Lehman CD, Harris JR, Pockaj BA, Khouri N, Hylton NF, et al. Statement of the science concerning locoregional treatments after preoperative chemotherapy for breast cancer: a National Cancer Institute conference. J Clin Oncol. 2008; 26:791–797. PMID:
18258988.

5. Gaffney DK, Tsodikov A, Wiggins CL. Diminished survival in patients with inner versus outer quadrant breast cancers. J Clin Oncol. 2003; 21:467–472. PMID:
12560437.

6. Lohrisch C, Jackson J, Jones A, Mates D, Olivotto IA. Relationship between tumor location and relapse in 6,781 women with early invasive breast cancer. J Clin Oncol. 2000; 18:2828–2835. PMID:
10920130.

7. Bräutigam E, Track C, Seewald DH, Feichtinger J, Spiegl K, Hammer J. Medial tumor localization in breast cancer: an unappreciated risk factor? Strahlenther Onkol. 2009; 185:663–668. PMID:
19806331.
8. Zucali R, Mariani L, Marubini E, Kenda R, Lozza L, Rilke F, et al. Early breast cancer: evaluation of the prognostic role of the site of the primary tumor. J Clin Oncol. 1998; 16:1363–1366. PMID:
9552038.

9. Keam B, Im SA, Kim HJ, Oh DY, Kim JH, Lee SH, et al. Clinical significance of axillary nodal ratio in stage II/III breast cancer treated with neoadjuvant chemotherapy. Breast Cancer Res Treat. 2009; 116:153–160. PMID:
18787948.

10. Farrús B, Vidal-Sicart S, Velasco M, Zanón G, Fernández PL, Muñoz M, et al. Incidence of internal mammary node metastases after a sentinel lymph node technique in breast cancer and its implication in the radiotherapy plan. Int J Radiat Oncol Biol Phys. 2004; 60:715–721. PMID:
15465187.

11. Heuts EM, van der Ent FW, von Meyenfeldt MF, Voogd AC. Internal mammary lymph drainage and sentinel node biopsy in breast cancer: a study on 1008 patients. Eur J Surg Oncol. 2009; 35:252–257. PMID:
18684584.
12. Turner-Warwick RT. The lymphatics of the breast. Br J Surg. 1959; 46:574–582. PMID:
13839973.

13. Huang O, Wang L, Shen K, Lin H, Hu Z, Liu G, et al. Breast cancer subpopulation with high risk of internal mammary lymph nodes metastasis: analysis of 2,269 Chinese breast cancer patients treated with extended radical mastectomy. Breast Cancer Res Treat. 2008; 107:379–387. PMID:
17457670.

14. Poortmans PM, Collette S, Kirkove C, Van Limbergen E, Budach V, Struikmans H, et al. Internal mammary and medial Ssupraclavicular irradiation in breast cancer. N Engl J Med. 2015; 373:317–327. PMID:
26200978.
15. Keam B, Im SA, Kim HJ, Oh DY, Kim JH, Lee SH, et al. Prognostic impact of clinicopathologic parameters in stage II/III breast cancer treated with neoadjuvant docetaxel and doxorubicin chemotherapy: paradoxical features of the triple negative breast cancer. BMC Cancer. 2007; 7:203. PMID:
17976237.

16. Chung Y, Kim JW, Shin KH, Kim SS, Ahn SJ, Park W, et al. Dummy run of quality assurance program in a phase 3 randomized trial investigating the role of internal mammary lymph node irradiation in breast cancer patients: Korean Radiation Oncology Group 08-06 study. Int J Radiat Oncol Biol Phys. 2015; 91:419–426. PMID:
25636764.

17. Kim WH, Han W, Chang JM, Cho N, Park IA, Moon WK. Location of triple-negative breast cancers: comparison with estrogen receptor-positive breast cancers on MR imaging. PLoS One. 2015; 10:e0116344. PMID:
25608004.

18. Lim ST, Choi JE, Kim SJ, Kim HA, Kim JY, Park HK, et al. Prognostic implication of the tumor location according to molecular subtypes in axillary lymph node-positive invasive ductal cancer in a Korean population. Breast Cancer Res Treat. 2016; 156:473–483. PMID:
27041335.

19. Tsai J, Bertoni D, Hernandez-Boussard T, Telli ML, Wapnir IL. Lymph node ratio analysis after neoadjuvant chemotherapy is prognostic in hormone receptor-positive and triple-negative breast cancer. Ann Surg Oncol. 2016; 23:3310–3316. PMID:
27401442.

20. Kim SH, Jung KH, Kim TY, Im SA, Choi IS, Chae YS, et al. Prognostic value of axillary nodal ratio after neoadjuvant chemotherapy of doxorubicin/cyclophosphamide followed by docetaxel in breast cancer: a multicenter retrospective cohort study. Cancer Res Treat. 2016; 48:1373–1381. PMID:
27034147.

21. Haffty BG, McCall LM, Ballman KV, McLaughlin S, Jagsi R, Ollila DW, et al. Patterns of local-regional management following neoadjuvant chemotherapy in breast cancer: results from ACOSOG Z1071 (Alliance). Int J Radiat Oncol Biol Phys. 2016; 94:493–502. PMID:
26867878.

22. Smith IC, Heys SD, Hutcheon AW, Miller ID, Payne S, Gilbert FJ, et al. Neoadjuvant chemotherapy in breast cancer: significantly enhanced response with docetaxel. J Clin Oncol. 2002; 20:1456–1466. PMID:
11896092.

23. Reitsamer R, Peintinger F, Prokop E, Hitzl W. Pathological complete response rates comparing 3 versus 6 cycles of epidoxorubicin and docetaxel in the neoadjuvant setting of patients with stage II and III breast cancer. Anticancer Drugs. 2005; 16:867–870. PMID:
16096435.

24. Gonzalez-Angulo AM, McGuire SE, Buchholz TA, Tucker SL, Kuerer HM, Rouzier R, et al. Factors predictive of distant metastases in patients with breast cancer who have a pathologic complete response after neoadjuvant chemotherapy. J Clin Oncol. 2005; 23:7098–7104. PMID:
16192593.

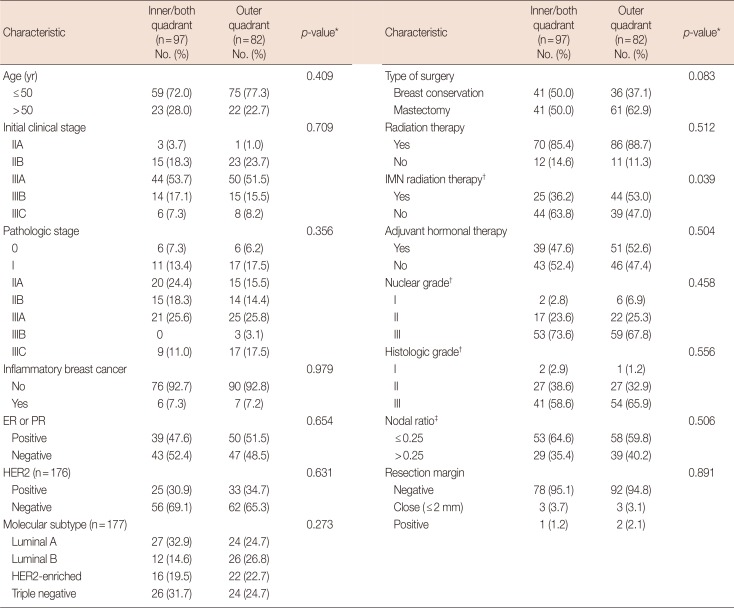
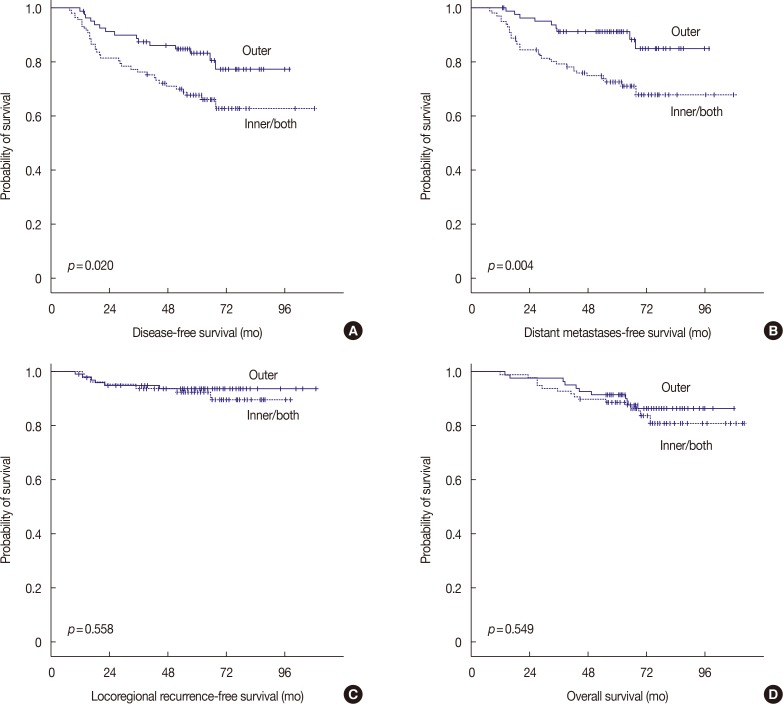
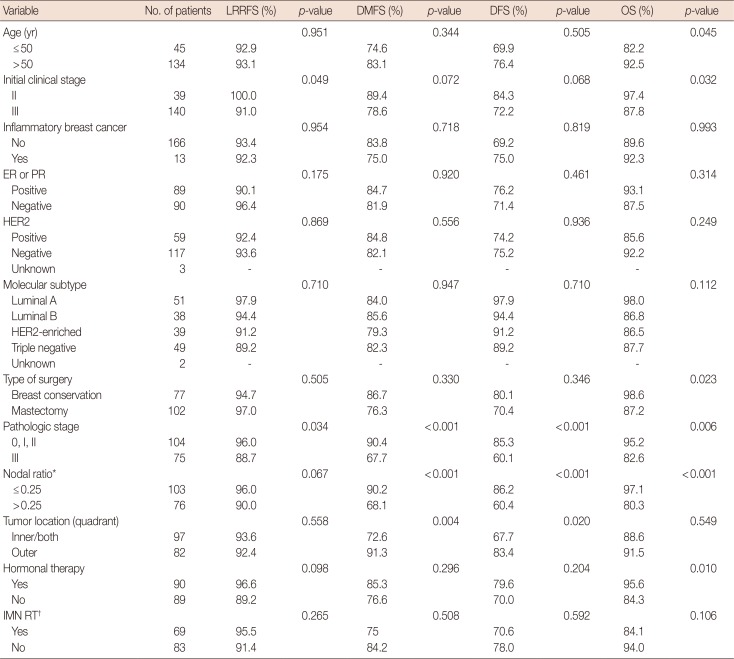
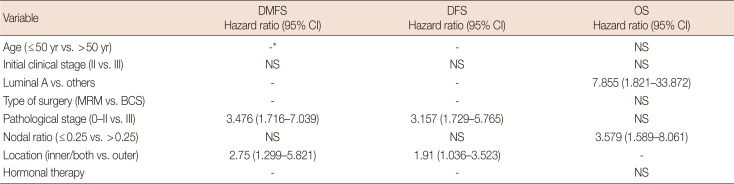




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download