Abstract
Purpose
Lipid rafts are cholesterol enriched microdomains that colocalize signaling pathways involved in cell proliferation, metastasis, and angiogenesis. We examined the effect of methyl-β-cyclodextrin (MβCD)-mediated cholesterol extraction on the proliferation, adhesion, invasion, and angiogenesis of triple negative breast cancer (TNBC) cells.
Methods
We measured cholesterol and estimated cell toxicity. Detergent resistant membrane (DRM) and non-DRM fractions were separated using the OptiPrep gradient method. Cell cycles stages were analyzed by flow cytometry, apoptosis was assessed using the TdT-mediated dUTP nick end-labeling assay, and metastasis was determined using a Matrigel invasion assay. Neo-vessel pattern and levels of angiogenic modulators were determined using an in vitro angiogenesis assay and an angiogenesis array, respectively.
Results
The present study found that the cholesterol-depleting agent MβCD, efficiently depleted membrane cholesterol and caused concentration dependent (0.1–0.5 mM) cytotoxicity compared to nystatin and filipin III in TNBC cell lines, MDA-MB 231 and MDA-MB 468. A reduced proportion of caveolin-1 found in DRM fractions indicated a cholesterol extraction-induced disruption of lipid raft integrity. MβCD inhibited 52% of MDA-MB 231 cell adhesion on fibronectin and 56% of MDA-MB 468 cell adhesion on vitronectin, while invasiveness of these cells was decreased by 48% and 52% respectively, following MβCD treatment (48 hours). MβCD also caused cell cycle arrest at the G2M phase and apoptosis in MDA-MB 231 cells (25% and 58% cells, respectively) and in MDA-MB 468 cells (30% and 38% cells, respectively). We found that MβCD treated cells caused a 52% and 58% depletion of neovessel formation in both MDA-MB 231 and MDA-MB 468 cell lines, respectively. This study also demonstrated that MβCD treatment caused a respective 2.6- and 2.5-fold depletion of tyrosine protein kinase receptor (TEK) receptor tyrosine kinase levels in both TNBC cell lines.
The plasma membrane is an indispensable barrier that separates the intracellular environment from a non-living environment. Conversely, it controls the movement of certain types of combinations in and out of the cell. Plasma membranes receive signals from both sides of the membrane that elicits an effect, resulting in the transmission of these signals, and a resulting cellular response. The cell membrane is a heterogeneous bilayer that has a disordered fluid state composed mainly of lipids, where proteins freely move within this “lipid sea” [1] and more ordered membrane domains that are enriched with cholesterol and sphingolipids. These ordered membranes within a lipid sea led to the concept of lipid raft membrane microdomains [2].
Lipid rafts are dynamic and detergent-insoluble plasma membrane microdomains that have been implicated in cell survival, proliferation, invasion, cell adhesion, protein sorting, and cholesterol metabolism [3]. A significant number of proteins involved in cancer development are associated with lipid rafts [4].
Lipid rafts are one of the most interesting characteristics of biological membranes as they can form unique localized domains with diverse compositions and physical properties [5]. Lipid rafts facilitate signal transduction cascades by recruiting signal proteins in response to intracellular and extracellular stimuli [6]. Lipid rafts are capable of limiting signaling either by physical sequestration of signaling components or by suppressing the intrinsic activity of signaling proteins present within the rafts [7].
Li et al. [8] demonstrated that cancer cells display higher levels of cholesterol-rich lipid rafts compared to those seen in normal cells. Recent studies have shown changes in the specific lipid molecules found in cancer cells as well as in the tumor microenvironment [9]. Accumulating evidence shows that cholesterol-rich rafts activate numerous proteins associated with cell survival including serine/threonine kinase 1 (AKT), insulin like growth factor 1 receptor (IGF1R), Fas cell surface death receptor (FAS) [3], and vascular endothelial growth factor receptors (VEGFR) [10].
Cholesterol is a key component of lipid rafts that accumulates more in cancer cells compared to normal cells [11]. Several epidemiologic studies have found a decreased incidence of certain cancers in patients taking statins, which inhibit the rate-limiting step of cholesterol biosynthesis [12]. These studies have proposed that there may be an association between cholesterol accumulation and risk for some cancers.
Disruption of lipid raft integrity reduces VEGFR2 dimerization and inhibits VEGFR2 endocytosis, signaling, and angiogenesis [13]. Lipid raft disruption affects homoeostasis by altering cellular survival and apoptotic pathways [14].
Cyclodextrins disrupt lipid raft integrity by sequestering cholesterol from the plasma membrane with a high affinity [15]. Methyl-β-cyclodextrin (MβCD) is an efficient acceptor and is commonly used to remove cellular cholesterol [16]. Filipin III disrupts lipid rafts by forming complexes with membrane cholesterol [17], while nystatin, which is a polyene antifungal drug, specifically sequesters membrane cholesterol, thereby reducing its ability to interact with other membrane components, such as receptors [18].
Breast cancer is a heterogeneous type of cancer and its genetic diversity is responsible for its rapid metastasis. The involvement of angiogenic pathways in metastasis of breast cancer subtypes has been reported for many years. Raghu et al. [19] demonstrated that lipid raft disruption reduced migration, invasion, and angiogenesis in breast cancer cells. However, the roles of angiogenic pathways in different subtypes of breast cancer or even within the same subtype may be different. Therefore, the purpose of our study was to investigate the effects of lipid raft disruption on the cellular mechanisms of proliferation, adhesion, invasion, and angiogenesis in two different triple negative breast cancer (TNBC) cell lines, MDA-MB 468 (drug-resistant) and MDA-MB 231 (drug sensitive), to better understand the cellular metastatic mechanisms that underlie breast cancer.
Human TNBC cell lines, MDA-MB 231 and MDA-MB 468 (NCCS, Pune, India) were maintained in advanced Dulbecco's modified Eagle medium (DMEM; Invitrogen, Carlsbad, USA), supplemented with L-glutamine (2 mM), 100 units of penicillin and streptomycin (Invitrogen), and 10% fetal bovine serum (Invitrogen). Human umbilical vascular endothelial cells (HUVEC; Invitrogen) were supplemented with Medium-200 and low serum growth supplements (Invitrogen) in a 5% CO2 incubator.
TNBC cells were treated with the lipid raft disrupting agents MβCD, nystatin and filipin III (Sigma, St. Louis, USA) at concentrations of 0.1, 0.2, 0.3, 0.4, and 0.5 mM (0.1–0.5 mM) for 1, 24, and 48 hours. Cellular cholesterol levels were measured using an Amplex® red cholesterol measurement assay (Invitrogen). Results were expressed as a percentage over controls.
Cell proliferation in terms cytotoxicity was measured using a lactate dehydrogenase (LDH) assay. TNBC cells were treated with different concentrations (0.1–0.5 mM) of MβCD, nystatin, and filipin III for 1, 24, and 48 hours. Supernatants were collected and assessed for cellular toxicity using the LDH Cytotoxicity assay kit (Invitrogen) in accordance with the manufacturer's instructions. Results were expressed as a percentage over controls.
Cells from both TNBC cell lines were pre-incubated in serum-free media and treated with 0.5 mM MβCD for 48 hours. Treated cells were subjected to 1% Triton X-100 followed by OptiPrep gradient separation to isolate detergent resistant membrane (DRM) and non-DRM fractions. A specific enzyme linked immunosorbent assay (ELISA) kit was used to analyze caveolin-1 (MyBioSource, San Diego, USA) and transferrin levels (Abcam, Cambridge, USA).
Cells were treated with 0.5 mM MβCD for 48 hours and cell cycle phases were analyzed using flow cytometry. Following treatment, cells were washed with 1× phosphate buffer saline (PBS), and incubated with 1 mL of propidium iodide (PI) for 30 minutes in the dark. DNA content was measured based on the presence of PI-stained cells. Flow cytometric analysis was performed using ≥10,000 cells from each sample with an excitation wavelength of 488 nm and an emission wavelength of 530 nm [20].
A TdT-mediated dUTP nick end-labeling (TUNEL) assay was performed for the rapid identification and quantification of apoptotic cells. Cells were treated with MβCD (0.5 mM) for 72 hours, and adherent cells were incubated with a reaction mixture containing biotin-2′-deoxyuridine 5′-triphosphate (biotin-dUTP) and terminal deoxynucleotidyl transferase for 1 hour. Cells were stained with 4′,6-diamidino-2-phenylindole (DAPI), mounted with cover slips, and positively fluorescein-labeled cells were visualized using fluorescent microscopy. Fluorescein-labeled cells were quantified and expressed as a percentage compared to DAPI-stained cells [20].
Cells were seeded at 2×104 cells/well in a 96-well plate precoated with the extracellular matrix (ECM) proteins type I collagen (5 µg/mL), fibronectin (2 µg/mL), vitronectin (2 µg/mL), and laminin-5 (4 µg/mL). Following 2 hours incubation at 37℃, cells were treated with MβCD (0.5 mM) for 48 hours. Nonadherent cells were removed by rinsing with PBS. Adherent cells were fixed and stained with Hema 3. Adherent cells from all treatment groups were counted and averaged for comparative quantification [21].
TNBC cells were either untreated or pretreated with 0.5 mM of MβCD for 1 hour, washed, and allowed to invade Matrigel-coated Transwell inserts (8 µM porosity; Corning Inc., Tewksbury, USA) for 48 hours. Cells which invaded the Matrigel-coated inserts were stained with Hema 3, counted, and photographed [21].
Cells from both TNBC cell lines were seeded in 100 mm plates and were either untreated or treated with 0.5 mM MβCD for 48 hours at 37℃. Following treatment, the medium was removed, washed, and serum-free medium was added. Conditioned medium was collected following overnight incubation. HUVEC cells (1×105 cells/well) were cultured in the conditioned medium for 24 hours. Following incubation, the medium was removed, cells were stained with Hema 3, and examined under a microscope. The extent of angiogenesis was measured by the number of branch points and the total number of branches per point [22].
MDA-MB 231 cells and MDA-MB 468 cells (1×105 cells/well) were treated with 0.5 mM MβCD and co-cultured with HUVEC (2×105 cells/well) for 48 hours. Untreated cells cocultured with HUVEC were maintained to serve as a control. Conditioned media was collected following overnight incubation, exposed to angiogenesis antibody arrays, and developed as per manufacturer's instructions (RayBiotech Inc., Norcross, USA). Angiogenic expression (measured as signal intensity) was quantified using densitometry while fold change was calculated by comparisons with the control [22].
Cells were treated with 0.5 mM MβCD for 48 hours followed by another 48-hour incubation with or without 1 mM cholesterol-MβCD complexes. Following treatment with cholesterol-MβCD complexes, cytotoxicity, cell adhesion and invasion, the proportion of cells in cell cycle phases, and the number of apoptotic cells, were measured as described previously [23].
Each experiment was carried out at least three times separately and the data were expressed as mean±SE. Statistical differences between control and target groups for all experiments were determined using Student t-test. The statistical significance was determined at 5 level (p<0.05).
We estimated the levels of cholesterol in normal (MCF 12A) and TNBC cell lines (MDA-MB 231 & MDA-MB 468), we found that TNBC cell lines exhibited higher ratios of cholesterol than the normal cell line (Supplementary Figure 1). To determine whether treatment of TNBC cells with different concentrations of MβCD, nystatin, and filipin III efficiently extracted cellular cholesterol, and to asses residual cholesterol levels 48 hours later, we assayed cellular cholesterol levels using an Amplex® Red Cholesterol Assay kit (Invitrogen). As shown in Figure 1A and B, extraction of cellular cholesterol increased with increasing MβCD concentration in a dose-dependent manner at 1, 24, and 48-hours in both cell lines. We observed a 58% and 56% reduction in cholesterol in MDA-MB 231 and MDA-MB 468 cells respectively, following a 48-hour exposure to 0.5 mM MβCD. We found a 32% reduction of cellular cholesterol levels in MDA-MB 231 and a 33% reduction in MDA-MB 468 cells using a 0.5 mM concentration of nystatin (Figure 1C and D), while a 48-hour exposure to filipin III resulted in a 29% and 30% reduction of cellular cholesterol levels cells from MDA-MB 231 and MDA-MB 468, respectively (Figure 1E and F). Thus, of the cholesterol sequestering agents assayed, 0.5 mM MβCD efficiently reduced cellular cholesterol in both cell lines with a 48-hour treatment.
Cholesterol sequestering agents are widely used to disrupt the integrity of lipid raft microdomains in plasma membranes. Cytotoxicity is typically measured to evaluate cell proliferation by quantifying plasma membrane damage. LDH, that is a cytosolic enzyme released into cell culture media, is an indicator of cellular toxicity. We evaluated the potential cytotoxicity of the cholesterol sequestering agents, MβCD, nystatin, and filipin III using the LDH cytotoxic assay. To determine the dose response, cells from both TNBC cell lines were treated with different concentrations of cholesterol sequestering agents for 1, 24, and 48 hours. MβCD caused 2%, 5%, 7%, 9%, and 12%; 13%, 20%, 29%, 44%, and 49%; 21%, 30%, 41%, 55%, and 63% of cytotoxicity after 1, 24, and 48 hours, respectively at 0.1 mM, 0.2 mM, 0.3 mM, 0.4 mM, and 0.5 mM concentration, respectively in MDA-MD 231 cells (Figure 2A). However, in MDA-MB 468 cells, MβCD caused 4%, 6%, 7%, 9%, and 11%; 13%, 19%, 28%, 39%, and 48% cytotoxicity at 1 and 24 hours, whereas 20%, 31%, 39%, 51%, and 61% at 48 hours (Figure 2B). Nystatin caused 2%, 4%, 6%, 7%, and 9%; 12%, 19%, 26%, 39%, and 41%; 18%, 29%, 32%, 42%, and 48% of cytotoxicity after 1, 24, and 48 hours treatment, respectively with 0.1, 0.2, 0.3, 0.4, and 0.5 mM concentration, respectively, in MDA-MD 231 cells (Figure 2C). However, in MDA-MB 468 cells, nystatin caused 2%, 5%, 9%, 10%, and 12%; 11%, 18%, 27%, 37%, and 46% of cytotoxicity at 1 and 24 hours, and 17%, 29%, 36%, 49%, and 55% at 48 hours (Figure 2D). Filipin III caused 1%, 2%, 3%, 5%, and 7%; 3%, 8%, 14%, 23%, and 30%; 5%, 12%, 21%, 39%, and 45% of cytotoxicity after 1, 24, and 48 hours, respectively with 0.1, 0.2, 0.3, 0.4, and 0.5 mM concentration respectively, in MDA-MD 231 cells (Figure 2E). However, in MDA-MB 468 cells, filipin III caused 1%, 2%, 5%, 9%, and 11%; 4%, 9%, 17%, 26%, and 32% of cytotoxicity at 1 and 24 hours, and 6%, 14%, 23%, 39%, and 47% at 48 hours (Figure 2F) (Table 1).
To further investigate the effect of MβCD on lipid rafts, DRM and non-DRM fractions were separated using an OptiPrep separation kit. Both fractions were analyzed for the specific marker proteins, caveolin-1 and transferrin, using ELISA. DRMs were found in the low-density fractions corresponding to the 5% to 35% sucrose interface. A significant proportion of caveolin-1 was found in the low density fraction of untreated cells, while a smaller proportion was seen in the same fraction in MβCD treated cells (Figure 3A and B). The nonraft protein, transferrin, was absent in DRM fractions of both treated and untreated cells but was found in the high-density DRM fraction of both TNBC cell lines (Figure 3C and D). Our findings confirm that MβCD efficiently extracts cholesterol from the lipid raft regions of the plasma membrane.
We also measured the effect of tamoxifen and cisplatin on MβCD induced cytotoxicity in triple negative breast cancer (TNBC) cell lines. After 48 hours of treatment, MβCD (0.5 mM) alone causes 63 and 61% cytotoxicity in MDA-MB 231 and 468 cells, respectively. Combination of MβCD (0.5 mM) with tamoxifen (5 µM) induces the increase in cell toxicity to 72 and 78% and MβCD (0.5 mM) with cisplatin (5 µM) shows increased cell toxicity to 76 and 82% in MDA-MB 231 & 468, respectively (Supplementary Figure 2).
Cancer metastasis involves cell adhesion, migration, and invasion. One of the primary events in metastasis is the attachment of tumor cells to the ECM, followed by proteolysis of the matrix. Therefore, to test the metastatic behavior of TNBC cells, we performed adhesion assays using the ECM proteins vitronectin, fibronectin, laminin, and type I collagen. Relative cell adhesion to each ECM protein was determined using BSA-induced adhesion as a control. The results showed that MDA-MB 231 cells significantly adhered to fibronectin, while MDA-MB 468 cells adhered to vitronectin (data not shown).
To study the effects of lipid raft disruption on fibronectin- and vitronectin-mediated adhesion of MDA-MB 231 cells and MDA-MB 468 cells respectively, cells from both cell lines were treated with 0.5 mM MβCD for 48 hours. We found that MβCD treatment markedly reduced cell adhesion in both cell lines on their respective ECM (Figure 4A). Quantification of our findings showed that 52% and 56% of cell adhesion in MDA-MB 231 and MDA-MB 468 respectively, was reduced following MβCD treatment (Figure 4B).
The unique feature of TNBC cells is a metastasis. Therefore, we investigated the effect of MβCD-mediated lipid raft disruption on breast cancer cell invasion using Matrigel. We found that lipid raft disruption significantly reduced invasive potential of MDA-MD 231 and MDA-MB 468 cells through Matrigel (Figure 4C) with MβCD treatment inhibiting cellular invasion by 48% and 52% in MDA-MD 231 and MDA-MD 468 cells respectively, compared to that seen in controls (Figure 4D).
To further study decreased metastasis of TNBC cells because of lipid raft disruption, we performed cell cycle analysis and found, based on cell distributions at different phases of the cell cycle, that G2M arrest occurred following exposure to 0.5 mM MβCD for 48 hours in cells from both TNBC cell lines (Figure 5A and B). Histogram analysis showed that the distribution of cells in the G2M phase in MDA-MB 231 cells was 5% in untreated and 25% in MβCD-treated (Figure 5C), while the distribution of cells in the G2M phase in MDA-MB 468 cells was 5% and 30% in untreated and MβCD-treated cells, respectively (Figure 5D).
Prolonged arrest of cells in the G2M phase may lead to apoptosis. Therefore, cells from both TNBC cell lines were treated with MβCD for 72 hours and apoptosis was evaluated using a TUNEL assay. We found that treatment with MβCD notably increased the number of TUNEL-positive cells in both cell lines (Figure 5E and F). Quantification of TUNEL-positive cells showed that 5% of untreated cells and 58% of MβCD-treated MDA-MB 231 cells were apoptotic (Figure 5G), whereas 8% of untreated cells and 38% of MβCD-treated MDA-MB 468 cells were apoptotic, as compared to the number of DAPI-stained cells which was set as 100% (Figure 5H).
The formation of new blood vessels from pre-existing vessels is vital during metastasis for the establishment and survival of cells in a new environment. A reduction of nutrients and oxygen supply to cancer cells by inhibiting neovessel formation may result in cell death. To gain insight into the role of lipid rafts in tumor-induced vessel formation, conditioned media collected from untreated and MβCD-treated MDA-MB 231 and MDA-MB 468 cells were used to culture HUVEC cells. Capillary-like networks developed when endothelial cells were cultured in conditioned media from untreated cells from either cell line. In contrast, reduced capillary network formation was seen when endothelial cells were cultured in conditioned media from MβCD-treated MDA-MB 231 and MDA-MB 468 cells (Figure 6A). We found that treatment of MDA-MB 231 cells with MβCD reduced endothelial capillary formation in cultured HUVEC cells by 52% compared to that found in controls. Interestingly, MDA-MB 468 cells treated with MβCD also showed decreased endothelial capillary formation in cultured HUVEC cells by 58% compared to that seen in controls (Figure 6B).
To evaluate the effect of lipid raft disruption on angiogenic modulators, human angiogenesis antibody array C1000 membranes were incubated with conditioned media collected from culturing HUVEC cells with untreated or MβCD-treated medium from MDA-MB 231 and MDA-MB 468 cell lines. We found that expression of tyrosine protein kinase receptor (TEK) was significantly decreased in both cell lines. Densitometry analysis showed that TEK expression decreased 2.6-fold in MDA-MB 231 cells (Figure 6C) and 2.5-fold in MDA-MD 468 cells compared to that seen in controls (Figure 6D).
To further confirm the role of lipid rafts in cell survival, cholesterol was supplemented to replenish the raft-associated cholesterol in MβCD-treated TNBC cells. We found that cell proliferation recovered by 90% to 95% with cholesterol supplementation in both TNBC cell lines (Figure 7A and B). To further confirm the role of lipid rafts in invasive behavior, raftassociated cholesterol was replenished in MβCD-treated TNBC cells. We found that 90% of MβCD-induced inhibition of adhesion and invasion of cells from both TNBC cell lines was recovered by cholesterol supplementation (Figure 7C and D). Furthermore, cholesterol supplementation abolished MβCD-induced cell cycle arrest (Figure 7E and F) and apoptosis (Figure 7G) in both MDA-MB 231 and MDA-MB 468 cells. These results show that lipid rafts are critical for the survival and metastasis of TNBC cells.
Lipid rafts of the plasma membrane are associated with a number of cellular processes. Recent studies have reported that diverse signaling molecules are present in lipid rafts, the disruption of which are known to affect signaling events [24]. Cholesterol, which is a key component of lipid rafts, is known to maintain the stability and architecture of the cell membrane.
Cholesterol accumulation has been reported in some cancers [11]. Li et al. [8] demonstrated that some breast cancer cell lines are more sensitive to lipid raft disruption than normal breast cell lines. However, the effect of lipid raft disruption by cholesterol depletion on TNBC survival has not been fully investigated.
In the present study, treatment of cells from the TNBC cell lines, MDA-MB 231 and MDA-MB 468, with different concentrations of the lipid raft disrupting agents MβCD, nystatin, and filipin III for different durations, showed reductions of cellular cholesterol in a dose- and time-dependent manner. Among the cholesterol depleting agents tested, MβCD at a concentration of 0.5 mM caused a significant reduction of cellular cholesterol after 48 hours of treatment compared to that seen with nystatin and filipin III. Our findings are in line with findings from our earlier studies [1923].
We also found that MβCD significantly inhibited cell proliferation in both TNBC cell lines in a dose- and time-dependent manner compared to that found using nystatin and filipin III. Li et al. [8] reported that MβCD inhibited proliferation of prostate and breast cancer cells in a dose-dependent manner, whereas their normal counterparts showed resistance to MβCD-induced cell death. Moreover, TNBC cells expressing epidermal growth factor receptor showed good migratory responses but a poor proliferative response to treatment. These studies indicate that identification of the key cellular mechanisms is required to effectively target TNBC.
Most breast cancer-related deaths are a result of metastasis, a process that involves a dynamic regulation of tumor cell adhesion and invasion. The adhesion of cancer cells to the ECM is critical for their survival, while disturbance of cell adhesion leads to the initiation of apoptosis [25]. In the present study, we found that MβCD treatment significantly reduced the adhesion of MDA-MB 231 cells onto fibronectin and MDA-MB 468 cells onto vitronectin. Tumor cell adhesion in the secondary site of specific organs is an essential part of the metastatic cascade and thus, the identification of inhibitors that prevent adhesion to specific matrix proteins represents potentially important therapeutic targets [26].
The aggressive nature and poor prognosis as well as the lack of an available targeted therapy makes TNBC clinically very challenging. Recently, Cheung et al. [27] identified cell invasion as an important step of metastatic progression in TNBC. Interestingly, we found that treatment of highly invasive TNBC cells from either MDA-MB 231 or MDA-MB 468 cell lines with MβCD significantly inhibited invasion.
Angiogenesis is the formation of new capillaries from a preexisting vascular network, and is essential for tumor growth and metastasis [28]. Furthermore, increased microvessel density in breast cancer is associated with lymph node metastasis. Lipid raft-associated VEGF receptors are critical players in angiogenesis [10]. In the present study, we found that disruption of lipid raft integrity by MβCD significantly reduced capillary formation in both TNBC cell lines as demonstrated using conditioned medium and the endothelial cell line, HUVEC. Raghu et al. [19] found that treatment of MDA-MB 231 cells with MβCD affected expression of the urokinase-type plasminogen activator receptor and matrix metalloproteinase-9, as well as had an effect on tumor-induced capillary formation. We demonstrated using an angiogenesis antibody array that MβCD treatment markedly decreased TEK expression in both TNBC cell lines. It was reported that TEK expression was higher in breast tumors compared to benign and normal breast tissues [29]. Our study shows that lipid rafts may regulate TNBC survival by modulating adhesion, invasion, and angiogenesis through TEK, and furthermore, demonstrates that lipid rafts have a pivotal role in cell survival in both TNBC cell lines.
In summary, disruption of lipid raft integrity by MβCD causes inhibition of cell proliferation in a dose- and time-dependent manner and furthermore, inhibits the cellular migratory response through prolonged arrest of the cell cycle in the G2M phase and by inducing apoptosis. We also found a marked decrease in TEK levels using an angiogenesis array, a finding that demonstrates a role of lipid rafts in regulating TEK levels in TNBC cells.
Figures and Tables
 | Figure 1Effect of cholesterol depleting agents on membrane cholesterol in triple negative breast cancer cells. MDA-MB 231 and 468 cells were treated with methyl-β-cyclodextrin (MβCD), nystatin and filipin III at different concentrations (0.1–0.5 mM) for 1, 24, and 48 hours and reduction in cellular cholesterol levels were measured. Reduction of cellular cholesterol with MβCD (A, B), nystatin (C, D) and filipin III (E, F) in MDA-MB 231 and MDA-MB 468 cells, respectively. The percent reduction in the cholesterol upon the treatments was calculated with respect to total cholesterol in the untreated cells, which was taken as 100%. The results represent the mean±SD of three independent experiments. |
 | Figure 2Effect of cholesterol depleting agents on cytotoxicity of triple negative breast cancer cells. MDA-MB 231 and 468 cells were treated with methyl-β-cyclodextrin (MβCD), nystatin and filipin III at different concentrations (0.1–0.5 mM) for 1, 24, and 48 hours and cell proliferation in terms of cytotoxicity was measured using lactate dehydrogenase assay. Cytotoxic effect of MβCD (A, B), nystatin (C, D) and filipin III (E, F) in MDA-MB 231 and MDA-MB 468 cells, respectively. The cytotoxicity was expressed as percent control. The results represent the mean±SD of three independent experiments. |
 | Figure 3Effect of lipid rafts disruption on detergent resistant membrane (DRM) and non-DRM fractions. The isolated DRM and non-DRM fractions were subjected to marker specific enzyme-linked immunosorbent assay. Levels of caveolin-1 in DRM and non-DRM fractions of untreated and methyl-β-cyclodextrin (MβCD) treated cells of MDA-MB 231 (A) and MDA-MB 468 (B). The transferrin levels in DRM and non-DRM fractions of untreated and MβCD treated cells of MDA-MB 231 (C) and MDA-MB 468 (D). |
 | Figure 4Effect of methyl-β-cyclodextrin (MβCD) on adhesion and invasion of triple negative breast cancer cells. (A) Adhesion assay was performed to evaluate the effects of MβCD on the adhesive potential of MDA-MB 231 and 468 cells to fibronectin and vitronectin-coated plates, respectively (stained with Hema 3, ×200). (B) Percent of adhesion was calculated from the mean obtained from three independent experiments and are represented (±SEM). (C) Transwell invasion assay was performed to evaluate the effects of MβCD on invasion of MDA-MB 231 and 468 cells through Matrigel (stained with Hema 3, ×200). (D) Percent of invasion was calculated from the mean obtained from three independent experiments and are represented (SEM). |
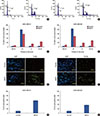 | Figure 5Effect of MβCD on cell cycle and apoptosis of triple negative breast cancer cells. Cell cycle distribution of MDA-MB 231 (A) and MDA-MB 468 cells (B). Propidium iodide stained cells were analyzed for DNA content using flow cytometry. Histograms represent the percentage of MDA-MB 231 (C) and MDA-MB 468 (D) cells in G0/G1, S and G2/M phases. The data represent one of three independent experiments. Values are mean±SD of three different experiments. MDA-MB 231 (E) and MDA-MB 468 (F) cells were stained for apoptosis using TdT-mediated dUTP nick end-labeling (TUNEL) assay. Quantification of apoptotic cells expressed as a percent of 4′,6-diamidino-2-phenylindole (DAPI)-stained cells in MDA-MB231 (G) and MDA-MB 468 (H). Data shown from three independent experiments, bars represent the mean±SD of three experiments. |
 | Figure 6Effect of Lipid raft disruption on tumor induced angiogenesis and expression of angiogenic molecules. In vitro angiogenesis in MDA-MB 231 and MDA-MB 468 cells (A). Tumor-induced tube formation in human umbilical vascular endothelial cells (HUVEC) cells was carried out as described in METHODS. The tube formation was observed under the bright field microscope and number of branch points were calculated (stained with Hema 3, ×400). (B) Graphical representation of relative branch points in MDA-MB 231 and MDA-MB 468 cells treated with methyl-β-cyclodextrin (MβCD). Bars represents the mean±SE of three different experiments. Expression of pro and antiangiogenic molecules in HUVEC and MDA-MB 231 or MDA-MB 468 co-cultures. Conditioned media from HUVEC and MDA-MB 231 or MDA-MB 468 co-cultures, exposed to angiogenesis antibody arrays and processed as per manufacturer's instructions. Graphical representation of fold change of pro- and antiangiogenic molecules (C, D). |
 | Figure 7Effect of cholesterol supplementation on survival and metastasis of methyl-β-cyclodextrin (MβCD) treated triple negative breast cancer cells. Cells were treated with MβCD for 48 hours and supplemented with cholesterol in the form of 1 mM MβCD-cholesterol complex for 24 hours. Proliferation of MDA-MB 231 cells (A) and MDA-MB 468 cells (B) in terms of cytotoxicity was measured using lactate dehydrogenase assay. Effect of cholesterol supplementation on adhesive potential of MDA-MB 231 and MDA-MB 468 (C) cells to fibronectin-coated and vitronectin-coated plates, respectively. Effect of cholesterol supplementation on Transwell invasion of MDA-MB 231 and 468 (D) cells through Matrigel and the percentage of MDA-MB 231 (E) and MDA-MB 468 (F) cells in G0/G1, S and G2/M phases. (G) Effect of cholesterol supplementation on TUNEL positive of MDA-MB231 and 468 cells. The values are expressed as means±SD of three independent experiments. |
Table 1
Effect of lipid raft disrupting agents on cell toxicity (%) of triple negative breast cancer cell lines

ACKNOWLEDGMENTS
The authors would like to thank DST-FIST, New Delhi, India. We also thank the authorities of GITAM University providing the facility for conducting this project.
References
1. Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972; 175:720–731.

3. Mollinedo F, Gajate C. Lipid rafts as major platforms for signaling regulation in cancer. Adv Biol Regul. 2015; 57:130–146.

4. Staubach S, Hanisch FG. Lipid rafts: signaling and sorting platforms of cells and their roles in cancer. Expert Rev Proteomics. 2011; 8:263–277.

8. Li YC, Park MJ, Ye SK, Kim CW, Kim YN. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am J Pathol. 2006; 168:1107–1118.

10. Caliceti C, Zambonin L, Rizzo B, Fiorentini D, Vieceli Dalla Sega F, Hrelia S, et al. Role of plasma membrane caveolae/lipid rafts in VEGF-induced redox signaling in human leukemia cells. Biomed Res Int. 2014; 2014:857504.

11. Zhuang L, Lin J, Lu ML, Solomon KR, Freeman MR. Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer Res. 2002; 62:2227–2231.
12. Marcella SW, David A, Ohman-Strickland PA, Carson J, Rhoads GG. Statin use and fatal prostate cancer: a matched case-control study. Cancer. 2012; 118:4046–4052.
14. George KS, Wu S. Lipid raft: a floating island of death or survival. Toxicol Appl Pharmacol. 2012; 259:311–319.

15. Ohtani Y, Irie T, Uekama K, Fukunaga K, Pitha J. Differential effects of alpha-, beta- and gamma-cyclodextrins on human erythrocytes. Eur J Biochem. 1989; 186:17–22.

16. Zidovetzki R, Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim Biophys Acta. 2007; 1768:1311–1324.

17. Bang B, Gniadecki R, Gajkowska B. Disruption of lipid rafts causes apoptotic cell death in HaCaT keratinocytes. Exp Dermatol. 2005; 14:266–272.

18. Pucadyil TJ, Shrivastava S, Chattopadhyay A. The sterol-binding antibiotic nystatin differentially modulates ligand binding of the bovine hippocampal serotonin1A receptor. Biochem Biophys Res Commun. 2004; 320:557–562.

19. Raghu H, Sodadasu PK, Malla RR, Gondi CS, Estes N, Rao JS. Localization of uPAR and MMP-9 in lipid rafts is critical for migration, invasion and angiogenesis in human breast cancer cells. BMC Cancer. 2010; 10:647.

20. Malla R, Gopinath S, Alapati K, Gondi CS, Gujrati M, Dinh DH, et al. Downregulation of uPAR and cathepsin B induces apoptosis via regulation of Bcl-2 and Bax and inhibition of the PI3K/Akt pathway in gliomas. PLoS One. 2010; 5:e13731.

21. Rao Malla R, Gopinath S, Alapati K, Gorantla B, Gondi CS, Rao JS. Knockdown of cathepsin B and uPAR inhibits CD151 and α3β1 integrin-mediated cell adhesion and invasion in glioma. Mol Carcinog. 2013; 52:777–790.

22. Malla RR, Gopinath S, Gondi CS, Alapati K, Dinh DH, Gujrati M, et al. Cathepsin B and uPAR knockdown inhibits tumor-induced angiogenesis by modulating VEGF expression in glioma. Cancer Gene Ther. 2011; 18:419–434.

23. Rao Malla R, Raghu H, Rao JS. Regulation of NADPH oxidase (Nox2) by lipid rafts in breast carcinoma cells. Int J Oncol. 2010; 37:1483–1493.

24. Mohammad N, Malvi P, Meena AS, Singh SV, Chaube B, Vannuruswamy G, et al. Cholesterol depletion by methyl-β-cyclodextrin augments tamoxifen induced cell death by enhancing its uptake in melanoma. Mol Cancer. 2014; 13:204.

25. Grossmann J. Molecular mechanisms of “detachment-induced apoptosis: Anoikis”. Apoptosis. 2002; 7:247–260.
26. Ghibelli L, Grzanka A. Organelle cross-talk in apoptotic and survival pathways. Int J Cell Biol. 2012; 2012:968586.

27. Cheung KJ, Gabrielson E, Werb Z, Ewald AJ. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell. 2013; 155:1639–1651.

Supplementary Materials
Supplementary Figure 1
Measurement of cholesterol levels in normal breast epithelial and triple negative breast cancer cell lines. MCF 12A and MDA-MB 231 and 468 cells were seeded in 96-well plate (8×103) in DMEM media for 48 hours. After overnight serum starvation, the cholesterol levels were measured in serum free medium using Amplex® red cholesterol measurement assay as described earlier. The amount of cholesterol was expressed µg/mL. The results represent the mean±SD of three independent experiments.
Supplementary Figure 2
Effect of tamoxifen and cisplatin on methyl-β-cyclodextrin (MβCD) induced cytotoxicity in triple negative breast cancer cell lines. MDA-MB 231 and 468 cells were treated with MβCD (0.5 mM) alone and in combination of MβCD (0.5 mM) with tamoxifen (5 µM) or MβCD (0.5 mM) with cisplatin (5 µM) for 48 hours and cytotoxicity was measured with lactate dehydrogenase assay. The cytotoxicity was expressed as percent. The results represent the mean±SD of three independent experiments.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download